Fig. 9.1
Compohe KineSpring® Knee Implant System (Moximed, Inc, Hayward, CA, USA). (a) Femoral base, (b) load absorber spring, (c) tibial base
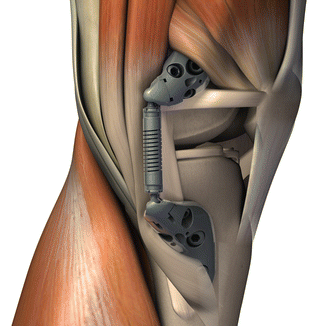
Fig. 9.2
Schematic drawing of the KineSpring® Knee Implant System (Moximed, Inc, Hayward, CA, USA) in relation to key anatomical structures
Concerns with this device include the durability and effectiveness of the spring mechanism and the potential for soft tissue irritation. The device is still in the clinical trial phase, but the initial clinical experience seems promising. Composite data from three clinical trials [13–15] in 99 patients with 17 months mean follow-up suggest excellent safety and effectiveness. All devices were successfully implanted and activated with no intraoperative complications. Statistically significant mean improvements of 56, 50, and 38 % were observed for Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain, Function, and Stiffness scores, respectively (all P < 0.001). WOMAC clinical success rates were 77.8 % for pain, 77.8 % for function, and 68.7 % for stiffness. The worldwide experience with the current generation KineSpring System has yielded favourable safety and durability outcomes with only 12 (8 %) patients undergoing device removal during follow-up for soft tissue impingement [6], return of OA symptoms [4], or deep infection [2]. Only one patient in this cohort was converted to arthroplasty after removal of the KineSpring device. Typical pretreatment and follow-up radiographs from this worldwide experience are shown in Fig. 9.3.
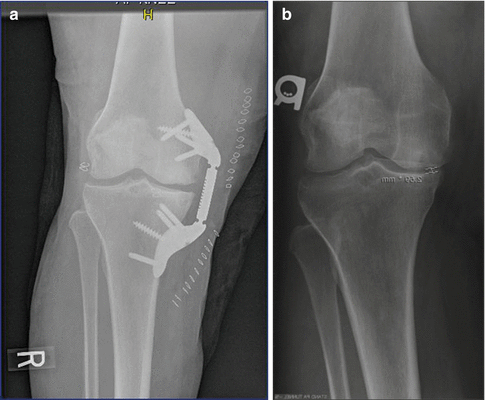

Fig. 9.3
(a) Preoperative anteroposterior radiograph showing pronounced osteoarthritis of the medial compartment. (b) The KineSpring® Knee Implant System (Moximed, Inc, Hayward, CA, USA) at 2 years post-implant
This type of device certainly appears to show promise and merits further investigation. Two clinical trials are currently underway to further evaluate the safety and effectiveness of the KineSpring System. The GOAL study (NCT01610505) 25 is a prospective, nonrandomized, controlled postmarket study comparing outcomes of 225 patients treated with the KineSpring System or high tibial valgus osteotomy. The first patient was enrolled in June 2012 and enrolment is expected to continue through 2013. A single-arm FDA-approved Investigational Device Exemption study ([SOAR] NCT01738165) [16] with 30 patients began enrolment in December 2012. Patient enrolment is anticipated to continue through mid-2013; the primary outcome will be evaluated at 2 years, and patients will be followed for 5 years post-treatment. The results of these clinical trials should help in deciding on the effectiveness and safety of these devices and in defining the appropriate indications.
9.4 Summary
Management of OA in the young active patient presents a significant challenge for the clinician who is trying to find appropriate options for patients who want to be active and not compromise their lifestyle, but avoid more invasive procedures such as arthroplasty. Procedures that are less invasive such as interposition arthroplasty and distraction or unloading arthroplasty potentially provide these less invasive options for patients that may not only improve symptoms but also positively affect the natural history of their condition.
Many of these devices, despite apparently well-considered designs, have ultimately had high failure rates when applied clinically. For this reason, any new technology needs to undergo appropriate scientific testing and clinical trials before introduction to the general community. The results of current clinical trials underway for devices discussed in this chapter will be awaited with interest. If early promising results translate to good results in clinical trials, then these devices may provide a useful addition to treatment options for these patients. Given the scope of this problem, it is inevitable that new technologies will continue to emerge with every year.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






