This article reviews the most common therapeutic and neuroprosthetic applications of neuromuscular electrical stimulation (NMES) for upper and lower extremity stroke rehabilitation. Fundamental NMES principles and purposes in stroke rehabilitation are explained. NMES modalities used for upper and lower limb rehabilitation are described, and efficacy studies are summarized. The evidence for peripheral and central mechanisms of action is also summarized.
Key points
- •
Hemiparesis following stroke is associated with significant upper and lower limb impairment, activity limitation, and reduced quality of life.
- •
Neuromuscular electrical stimulation as a motor relearning tool reduces upper and lower limb motor impairment following stroke.
- •
Neuromuscular electrical stimulation as a neuroprosthesis improves ambulation function of stroke survivors but not more than the standard of care ankle-foot orthoses.
- •
Research is needed to more firmly establish the effects of electrical stimulation on upper limb activity limitations and quality of life.
- •
The benefit of upper limb neuromuscular electrical stimulation modalities relative to alternative therapies or standard of care remains to be fully elucidated.
Introduction
Motor impairment is common after stroke and directly impacts the stroke survivor’s function and quality of life. Neuromuscular electrical stimulation (NMES) may reduce disability by improving recovery of volitional movement (therapeutic effect) or by assisting and replacing lost volitional movement (neuroprosthetic effect). This article describes NMES treatment modalities for upper and lower limb stroke rehabilitation and summarizes the research literature regarding the therapeutic and neuroprosthetic efficacy of those modalities. The scope of this article is limited to NMES interventions that produce limb movement by direct stimulation of the peripheral nerves or motor points of target muscles for the purpose of restoring motor function and, therefore, does not cover somatosensory electrical stimulation, electrical stimulation for poststroke shoulder pain, or brain stimulation modalities.
Introduction
Motor impairment is common after stroke and directly impacts the stroke survivor’s function and quality of life. Neuromuscular electrical stimulation (NMES) may reduce disability by improving recovery of volitional movement (therapeutic effect) or by assisting and replacing lost volitional movement (neuroprosthetic effect). This article describes NMES treatment modalities for upper and lower limb stroke rehabilitation and summarizes the research literature regarding the therapeutic and neuroprosthetic efficacy of those modalities. The scope of this article is limited to NMES interventions that produce limb movement by direct stimulation of the peripheral nerves or motor points of target muscles for the purpose of restoring motor function and, therefore, does not cover somatosensory electrical stimulation, electrical stimulation for poststroke shoulder pain, or brain stimulation modalities.
Neuromuscular electrical stimulation fundamentals
NMES is the use of electrical current to produce contractions of paralyzed or paretic muscles. Lower motor neurons to target muscles must be intact for NMES to effectively produce muscle contractions; therefore, NMES is usually only applicable to patients whose paralysis or paresis is caused by upper motor neuron injury (eg, stroke, spinal cord injury, and so forth). NMES can be applied to paretic muscles with surface electrodes positioned on the skin over the motor points of target muscles or with electrodes that are implanted near or on the muscle motor points or nerves that innervate target muscles. The electrical current generated by most NMES devices can be characterized as a waveform of pulses having a particular pulse frequency, width, and amplitude. Adjusting the pulse parameters can modulate the strength of evoked muscle contraction. Typically, the stimulation frequency is set between 12 and 50 Hz; the strength of the muscle contraction is modulated by changing either the pulse amplitude (typically 0–100 mA) or pulse width (typically 0–300 μs).
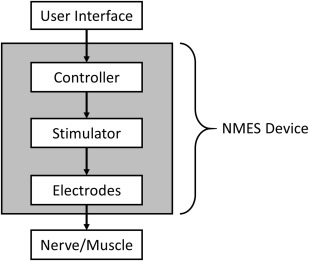
An NMES device fundamentally consists of electrodes that are connected to a stimulator and a controller ( Fig. 1 ). A pair of electrodes constitutes a stimulus channel. Surface (ie, transcutaneous) electrodes, percutaneous intramuscular wire electrodes, and implanted epimysial, intramuscular or nerve cuff electrodes may be used. The stimulator (ie, pulse generator) may have a controller built into it or have a separate controller attached or wirelessly linked to it. The controller regulates the timing and intensity of stimulation delivered through one or multiple stimulus channels. Input to the stimulator’s controller may be via buttons, switches, and/or various types of external or implanted sensors or recording (eg, electromyographic) electrodes.
Purposes of neuromuscular electrical stimulation for upper and lower limb rehabilitation after stroke
Paresis is the inability or decreased ability to volitionally activate motor units and is one of the most common manifestations of stroke. Clinically, paresis presents as muscle weakness and reduced speed of activation and the inability to generate functionally useful movement of the involved limb. Lang and associates studied the relative strengths of the associations between specific upper limb impairments and function and concluded that paresis was the strongest contributor to the loss of function. In the upper limb, the combination of paresis, loss of fractionated movements, flexor hypertonia, and somatosensory abnormalities often manifest as difficulty extending the elbow and opening the hand in a functional manner, which severely limits the functional work space. At 6 months after a stroke, about 65% of patients still cannot incorporate the affected arm and hand into their daily activities. Therefore, NMES for upper limb stroke rehabilitation is usually applied to the elbow, wrist, and/or hand extensor muscles.
In the lower limb, paresis, along with the inability to grade muscle contractions, poor motor coordination, poor endurance, spasticity, and impaired balance have significant consequences on ambulation. At 6 months after a stroke, approximately 30% of stroke survivors are unable to walk unassisted. A major contributor to impaired ambulation is the inability to dorsiflex the ankle during the swing phase of the gait. Diminished ankle dorsiflexion, knee flexion, or hip flexion can result in the inability to clear the floor with the affected limb during the swing phase of the gait, resulting in difficult and unsafe ambulation or nonambulation. Patients frequently use compensatory strategies, such as circumduction, hip hiking, or vaulting, to clear the toes. An ankle-foot orthosis (AFO) is the standard of care for foot drop; but because AFOs limit ankle mobility, they may actually inhibit recovery of dorsiflexion. Therefore, NMES has been used to improve ankle dorsiflexion and a more normal gait pattern.
Various NMES modalities have been used for upper and lower limb motor relearning after a stroke. Motor relearning is defined as the reacquisition of motor skills following central nervous system injury. NMES can be used as a motor relearning tool by enabling stroke survivors with significant paresis to participate in goal-oriented repetitive movement therapy. The NMES-mediated task must be repetitive, novel, volitionally controlled, and functionally relevant. Although stroke survivors may use an NMES motor relearning system to assist execution of daily activities, its primary intent is training, such that improved functional use of the hemiparetic limb is maintained when the system is not being used . Improved upper limb function or ambulation that remains after an NMES device has been used is called a therapeutic effect .
For patients who are in the chronic phase of stroke and in whom motor relearning strategies have been exhausted, NMES may be used as a neuroprosthesis. The primary intent of a neuroprosthesis is to enable patients to execute functional tasks with the affected upper limb or walk while using the device as part of routine daily living. Improved function that is realized while using an NMES device is called a neuroprosthetic effect .
Neuromuscular electrical stimulation modalities for upper limb rehabilitation
Cyclic NMES uses a 1- or 2-channel stimulator to activate the wrist and/or finger and thumb extensors in a repetitive (cyclic) fashion via surface electrodes placed on the forearm over the motor points of those muscles. Cyclic NMES devices typically have a menu of on/off cycle settings from which to choose. Once the device is set up and switched on, the stimulation automatically ramps on and off according to a selected duty cycle, with patients not having to exert any simultaneous effort. Patients do not control the timing or intensity of cyclic stimulation ( Table 1 ); therefore, this modality is not typically used to mediate functional task practice.
| Patients Have Real-time Control of the Following: | Cyclic NMES | EMG-Triggered NMES | Switch-Triggered NMES | Contralaterally Controlled NMES |
|---|---|---|---|---|
| Timing of NMES | No | Onset only | Yes | Yes |
| Intensity of NMES | No | No | No | Yes |
Cyclic NMES has been shown in several randomized controlled trials (RCTs) of acute and subacute hemiplegic patients to reduce upper limb motor impairment (eg, increase in strength, upper limb Fugl-Meyer score, and so forth) relative to controls. Some studies reported an enduring effect over 2 to 6 months, whereas others found that the effect was not sustained beyond the treatment period. Some studies found that the positive effects on impairment did not translate to significant improvements in basic self-care tasks or upper limb function (ie, functional independent measure score, action research arm test [ARAT]) relative to controls, whereas other studies did show significant, though sometimes transient, improvements in function relative to controls. The beneficial effects of cyclic NMES seem to be more apparent in patients who have some residual movement at baseline. In a study of 95 subacute patients, initial motor severity (ie, baseline Fugl-Meyer score) was identified as the most significant predictor of improvement in upper limb function after 4 weeks of cyclic NMES. Studies of cyclic NMES in chronic hemiplegia have typically been relatively small case series designs (ie, no control group) but have also demonstrated improvements in various upper limb motor impairment measures.
Electromyographic (EMG)-triggered NMES attempts to make stimulated hand opening coincide with the patients’ own effort to open the hand. Surface EMG recording electrodes are placed over the wrist and/or finger extensors of the paretic side to detect EMG signals when patients attempt to open the hand. When the processed EMG signal surpasses a preset threshold, electrical stimulation ramps on to a preset stimulation intensity that produces full hand opening. After several seconds, the stimulation turns off and the patients are prompted with visual and/or audio cues to try to open the hand again, repeating the EMG-triggered NMES cycle. Thus, EMG-triggered stimulation facilitates repetitive and volitionally initiated exercises of the hemiparetic upper extremity and provides cutaneous and proprioceptive feedback time-locked to each attempted movement, which may be important for motor relearning. Like cyclic NMES, EMG-triggered NMES is not typically used to mediate functional task practice because the intensity and duration of stimulation are not controlled by the patients (see Table 1 ). Because EMG-triggered NMES requires patients to be able to produce discernable EMG signals consistently, it may not be applicable to the most severely impaired patients.
EMG-triggered NMES has been shown to improve upper limb motor impairment . An early case series study of 69 chronic patients reported improvement in wrist active range of motion and extensor EMG activity in response to EMG-triggered NMES integrated with conventional therapy. The participants who received a greater dosage (ie, sessions per week) of EMG-triggered NMES had greater increases in voluntary extensor EMG amplitude. RCTs in chronic hemiplegia also show that EMG-triggered NMES improved performance on one or more measures of motor impairment (eg, Fugl-Meyer score, Box and Blocks score, extensor and grip strength) as compared with conventional therapy, though not all studies agree on which outcomes improve relative to controls. Most of the trials in chronic patients did not assess upper limb function (ie, activity limitation) or the persistence of effect. In acute and subacute patients, an RCT showed greater improvement on impairment measures but not on upper limb function relative to conventional therapy ; but another study showed the opposite: improvement on function (ie, ARAT) but not on impairment measures relative to usual care. Nearly all of the RCTs of EMG-triggered NMES have had small sample sizes (ie, <10 per group); like cyclic NMES, the improvements relative to controls are generally modest and of questionable clinical relevance.
Although EMG-triggered NMES might be expected to improve upper limb movement and function more than cyclic NMES, several RCTs that directly compared the two treatments showed no significant difference in the outcomes of cyclic and EMG-triggered NMES, whether in chronic or subacute subjects. Explanations for why no differences in outcomes were found between cyclic and EMG-triggered NMES include: (1) EMG-triggered NMES may not require enough active involvement (ie, patients only trigger stimulation, not control duration or intensity) to create a large enough contrast with cyclic NMES. (2) The cyclic NMES group may have also been exerting effort during stimulation, further reducing the contrast between the two treatments. (3) With EMG-triggered NMES, any time delays between the attempt to extend the wrist and fingers and the initiation of stimulation may negate any neurophysiologic advantage the treatment might have had over cyclic NMES.
Switch-triggered NMES is a modality intended to facilitate functional task practice. Switches (or button presses) allow patients or the therapist to control both the initiation and termination of stimulation sequences (ie, the timing of the stimulated movement; see Table 1 ) with button presses so that the device can be used in assisting task practice during therapy sessions. The intensity of stimulation is not controlled by patients but is preset. The Bioness H200 (Bioness Inc, Valencia, CA) is an example of a switch-triggered device that stimulates finger and thumb extensors and flexors through surface electrodes that are mounted inside a wrist-forearm orthosis, which also houses the stimulator. Patients turn the stimulation on and off to the extensors and flexors by pressing buttons on a separate control unit with their unaffected hand. Stimulation sequences that produce different hand opening and closing postures can be programmed and selected to match the task to be performed. Significant therapeutic effects were reported on several measures of motor impairment (eg, Box and Blocks score, Ashworth score) and function (eg, timed Jebsen-Taylor Hand Function tasks) in chronic patients after 5 weeks of home exercise and task practice with the Bioness H200. Several follow-up RCTs in acute and subacute patients found that switch-triggered NMES with therapy had greater improvements than therapy alone on measures of spasticity, wrist extension, Box and Blocks score, Fugl-Meyer score, and timed tasks. Although the Bioness H200 can be used as a neuroprosthesis and has been shown to have a significant neuroprosthetic effect, it is typically used and studied as a motor relearning tool.
Another switch-triggered NMES approach uses stimulation only as needed to assist first with repetitive reaching tasks (stimulating shoulder and elbow muscles) and then with grasping tasks (stimulating wrist, finger, and thumb muscles), progressively decreasing the use of NMES as patients improve. The treating therapist uses button switches to activate the stimulation sequences that are needed to perform tasks. Greater therapeutic effects were measured in acute patients who had 12 to 16 weeks of this switch-triggered NMES approach as compared with patients who received conventional task-specific occupational therapy.
Sensor- and EMG- controlled NMES modalities use controllers that are designed to let patients control the timing and intensity of stimulation to their hand in a way that can be fluid with task practice, which may result in greater sensorimotor integration and superior motor relearning (ie, therapeutic effects). Such systems may also be suitable as neuroprostheses to assist with activities of daily living. Indeed, the earliest NMES devices for upper limb stroke rehabilitation used a sensor mounted to the contralateral shoulder to let patients proportionally control the intensity of stimulation to the forearm extensors as they practiced tasks. Electrogoniometers, bend sensors, touch-sensitive mats, and accelerometers are among the external sensors that have been incorporated into NMES systems for upper limb stroke rehabilitation. Researchers also continue to explore the use of EMG signals from the impaired upper limb to not merely trigger the onset of a preset intensity and duration of stimulation but also to control the intensity and timing of stimulation. A challenge for EMG-controlled NMES modalities is that the effort required from patients to contract the muscle that operates the controller may induce flexor synergies or hypertonia, which can overpower the electrical stimulation of extensors and result in reduced degrees of stimulated hand opening.
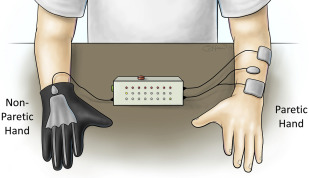
Contralaterally controlled NMES is a unique version of sensor-controlled stimulation that uses movement from the unimpaired side to control the timing and intensity of stimulation to the paretic side (see Table 1 ). The hand system consists of a glove with bend sensors worn on the nonparetic hand and a multichannel stimulator that delivers stimulation to the paretic hand extensors with an intensity that is proportional to the degree of opening of the glove ( Fig. 2 ). This modality enables repetitive hand opening exercise and functional task practice with the paretic hand. The control strategy gives the user intimate proportional control of the stimulation intensity without requiring any residual movement or EMG signals from the paretic hand. Therefore, the likelihood of triggering flexor synergy patterns may be less than sensor-controlled or EMG-controlled stimulation devices that require control signals from the paretic limb. Contralaterally controlled NMES produced larger improvements in maximum voluntary finger extension and other measures of upper extremity impairment and activity limitation than cyclic NMES in an RCT of subacute patients.
Neuromuscular electrical stimulation modalities for lower limb rehabilitation
Cyclic, EMG-triggered, and contralaterally controlled NMES applied to paretic lower limb muscles while patients are seated or side-lying has been evaluated for therapeutic effects. In a randomized placebo-controlled trial of 46 acute hemiplegic subjects, cyclic NMES was applied to the quadriceps, hamstring, tibialis anterior, and medial gastrocnemius in an activation sequence that mimicked normal gait while the subjects were side-lying with their lower extremity supported by a sling. Significantly greater improvement in ankle dorsiflexion torque and EMG activity and significantly less spasticity and cocontraction were demonstrated after 3 weeks of multichannel cyclic NMES as compared with the control group. Also, a significantly greater percentage of subjects in the cyclic NMES group were able to complete a timed walking task by the end of the 3-week treatment and 5 weeks later as compared with the control group. EMG-triggered NMES of paretic ankle dorsiflexors has been shown to have positive effects on ankle strength, range of motion, balance, and ambulation in chronic patients. Contralaterally controlled NMES, whereby patients controlled the intensity of stimulation to the paretic ankle dorsiflexors by dorsiflexion of their nonparetic ankle while seated, was first tested in a case series and later in an RCT. Contralaterally controlled NMES was shown to increase lower extremity Fugl-Meyer score, maximum dorsiflexion angle and moment while seated, and performance on the modified Emory Functional Ambulation Profile in chronic patients, but not more than cyclic NMES.
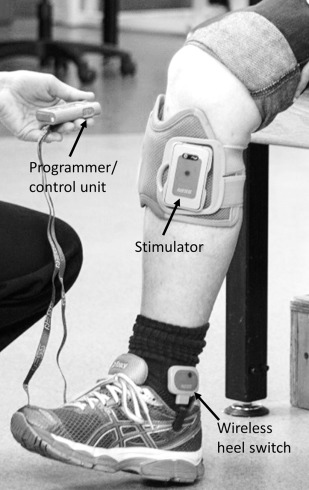
Liberson and associates first describes applying NMES to the paretic ankle dorsiflexors (ie, peroneal nerve) during the swing phase of the gait in 1961. The common peroneal nerve was stimulated with a pair of electrodes, one placed just below the head of the fibula and the other over the tibialis anterior. A heel switch worn in the shoe of the paretic side turned the stimulation on when the foot was lifted off the ground and turned the stimulation off at heel strike and during the stance phase of the gait. Currently, there are 3 surface electrode NMES systems for preventing foot drop during gait approved by the Food and Drug Administration: the Odstock Dropped-Foot Stimulator (Odstock Medical Limited, Salisbury, UK), WalkAide (Innovative Neurotronics Inc, Austin, TX), and Bioness L300 Foot Drop System (Bioness Inc, Valencia, CA). These devices use either a heel switch or a tilt sensor below the knee to synchronize the timing of stimulation to the swing phase of the gait ( Fig. 3 ). Two multichannel foot-drop systems with implanted electrodes and stimulator have the CE mark in Europe. One is a dual-channel device developed by the University of Twente (Netherlands) that stimulates the deep and superficial branches of the common peroneal nerve for better control of ankle dorsiflexion, eversion, and inversion. The other is a 4-channel device developed at Aalborg University (Denmark) and uses a 4-channel nerve cuff electrode surgically placed around the common peroneal nerve.