Chapter 6 Neuromuscular dysfunction in whiplash associated disorders
The pain and trauma of a whiplash injury cause changes in muscle properties and in neuromuscular function. This chapter explores what is known of these reactions and their potential interactions with other features associated with a whiplash injury. Such knowledge underpins a research-informed rationale and approach to rehabilitation of neuromuscular dysfunction.
Changes in the properties of the cervical muscles
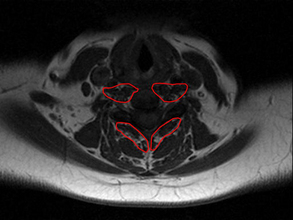
Imaging studies (e.g. ultrasound and magnetic resonance imaging [MRI]) have shown alterations in the physical characteristics (i.e. fatty infiltrates, changes in cross-sectional area) in the cervical extensor and flexor muscles in patients with chronic whiplash-related neck pain.1–3 These changes are present in both the neck extensors and flexors,2, 3 and are most notable in muscles that possess a larger proportion of type I, slow-oxidative fibres and a higher density of muscle spindles (e.g. the deep longus colli/capitis, suboccipital muscles and multifidus) (Fig 6.1).4, 5
In relation to fatty infiltrates, the mechanism(s) of the traumatic whiplash injury event itself may underlie the changes in the muscles in some patients. The fatty replacement in the cervical extensor muscles was present in those with persistent symptoms following whiplash but not in individuals with persistent insidious-onset neck pain or in those without neck pain.2, 6, 7 The degree of fatty infiltrate was not associated with age, body mass index, the duration of the disorder or compensation status, suggesting that the traumatic event may be a potential trigger for the changes.
While trauma may be a trigger, a preliminary investigation has shown that sensory, physical, kinaesthetic and psychological features all contribute to some extent to explain the varying levels of fatty infiltrate in muscle tissue.8 These features characterise a complex, non-resolving injury and some are prognostic of non-recovery.9 Of note, regression analysis determined that decreased cold pain threshold was a significant factor in the predictive model (r2 = 0.28; P = 0.02), and this may provide new insights into the clinical presentation of the chronic condition. Hyperalgesia to cold stimuli, which has been identified in several investigations of WAD, may relate to alterations in the central nervous system’s processing of pain.10–13 These centrally driven changes, possibly indicative of neuropathic pain, may be potentially irreversible.14 Thus, identifying quantifiable MRI changes in muscle properties and relating them to prognostic clinical measures in the chronic state could provide some plausible clinical hypotheses for possible pathophysiological processes of recalcitrant WAD.
A number of potential mechanisms could explain the changes in muscle structure and subsequent alterations in neuromuscular behaviour. Such factors include, but are not limited to: generalised disuse,15 chronic denervation,16, 17 systemic or inflammatory myopathy following trauma18, 19 or involvement of the sympathetic nervous system.20
Disuse
With disuse, morphometric changes in muscles are not uniform. For example, greater changes have been shown in muscles with more type I, slow-oxidative fibres.21–24 This could account for the larger fat infiltration in the deep cervical muscles,6, 8 which feature a greater density of type I fibres.5 Disuse is, thus, a plausible hypothesis for muscle degeneration in whiplash-injured people with a long duration of symptoms. Nevertheless, recent evidence may refute disuse as the primary mechanism for long-term muscle alterations, as patients with chronic non-traumatic neck pain, despite having a greater mean duration of pain than those in a chronic whiplash group, showed values of fatty infiltrate that mirrored those with no history of neck pain.6 Nevertheless, disuse may be more closely related to higher levels of pain rather than duration of symptoms. Pain levels may profoundly affect activity levels and, thus, muscle composition. Further investigations of people with pain and disability levels comparable to chronic whiplash are required to draw more definitive conclusions.
Response to trauma
The fatty changes observed in muscle may be adipoplastic or myoblastic in origin.16 In particular, fibroblasts and pre-adipocytes are present in the perimuscular connective tissue and these precursor cells can differentiate into adipose tissue in response to traumatically induced local inflammation.25, 26 Such a hypothesis follows the results of investigations in animals by Gradl et al.27, 28 Profound changes in muscle tissue, including myocyte apoptosis, were observed in animals with a more severe injury and subsequently greater inflammatory reactions.27, 28 Animals with a lesser injury did not exhibit such muscle changes. It is plausible that the changes (fatty infiltrates) in the neck muscles of patients with chronic whiplash may represent a more severe injury with greater motor impairments.
Muscular response to nerve injury
Until recently, it has been difficult to explain the specific mechanisms by which injury to different anatomical tissues in the spine alters the composition of the paraspinal muscles. Hodges et al.29 demonstrated differential and rapid muscular changes within three days following experimental nerve and disc lesions. Specifically, this study demonstrated rapid ipsilesional and monosegmental atrophy and fat proliferation in the porcine lumbar multifidus with a disc lesion. In contrast, with injury to the medial branch of the third lumbar dorsal ramus, rapid atrophy and fat tissue were observed bilaterally and in multisegments of the multifidus muscle. These results provide evidence of rapid and differential alterations to the lumbar paraspinal muscles following induced injury to different anatomical tissues. It is possible that similar time frames and patterns for fat development occur in some patients following traumatic whiplash injury and, as such, could implicate specific anatomical tissues.
The segmental posterior cervical muscles receive their nerve supply from dorsal branches that are most proximal to the spinal nerve root.30, 31 Thus, injury that is proximal to the origin of the dorsal branch may denervate the posterior paraspinal muscles. However, denervation of these muscles is unlikely if the injury is distal to the origin of the dorsal branch.30, 31 An interesting aspect of the study by Hayashi et al.30 was that abnormal MRI enhancement of the multifidus muscle did not occur without abnormal findings in the more superficial paraspinal muscles and without root avulsion. The pattern of fatty infiltrate in a chronic whiplash cohort (greater muscle changes in the deep multifidi compared to the superficial muscles2) reflects that of Hayashi et al.30 While the cause of symptoms in whiplash is not likely to be due to nerve root avulsion, the multisegmental paraspinal fatty infiltrates in the multifidi together with the presence of altered pain processing in some subjects with chronic symptoms may implicate a lesser injury that was either proximal or just distal to the dorsal root ganglion prior to the ‘take-off’ of the medial branch of the dorsal ramus. Animal studies are required to test this hypothesis, whereby lesions can be made to specific anatomical structures and their effects on the muscular system can be controlled.
The possible diverse mechanisms for muscular alterations highlight a potentially confusing pathological presentation. The relationship between neck muscle degeneration, pain and disability levels and clinical features in the acute whiplash state must be confirmed or refuted before drawing strong clinical conclusions. Current and future research with advanced imaging should not assume a direct pathoanatomical correlate with symptoms. Rather, efforts should be placed on establishing whether the temporal development and the magnitude and location of neck muscle changes are related to clinical features (i.e. range of movements and neuromuscular functioning) and varying levels of pain and disability.
Altered neuromuscular control in WAD
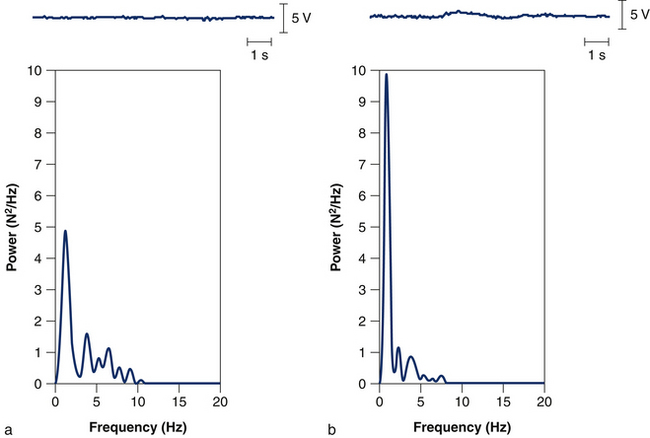
In addition to changes in muscle properties, changes occur in neuromuscular control. Individuals who have experienced a whiplash injury are known to have impaired neck strength, which is apparent around all axes.32 Furthermore, they show an impaired ability to maintain a steady neck contraction around a target force value.33 Reduced force steadiness in patients with whiplash has been attributed to alterations in group Ia afferent input as these patients show greater power in the low frequency band of the force signal compared to asymptomatic subjects (Fig 6.2). Altered cervical afferent input may result from damaged or functionally impaired neck mechanoreceptors,34 sensitisation of mechanoreceptors from pain,35 decreased central inhibition14 and sympathetic effects on muscle spindle sensitivity.20 Accordingly, short-term vibration of the neck, which stimulates group Ia afferent input, increases force steadiness in women with whiplash-induced neck pain.33
Changes in motor output are accompanied by alterations in muscle strategies. Evidence suggests that neck pain may alter the task-related modulation of neck muscle activity so that motor control of the cervical spine is solved by alternative, presumably less efficient, combinations of muscle synergistic activities. As an example, impaired performance on a task of craniocervical flexion (anatomical action of the deep longus colli and longus capitis) has been shown to be a feature of whiplash-induced neck pain36–38 and is present in other neck pain disorders, such as idiopathic neck pain,39 work-related neck pain40 and cervicogenic headache.41 Reduced activation of the deep cervical flexors is associated with increased activation of the superficial neck muscles in patients with neck pain,39 which is known to occur within the first four weeks of a whiplash injury.36
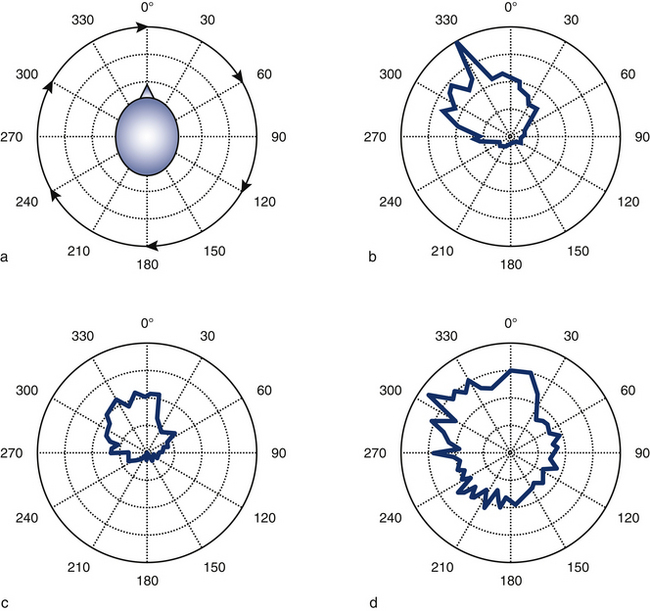
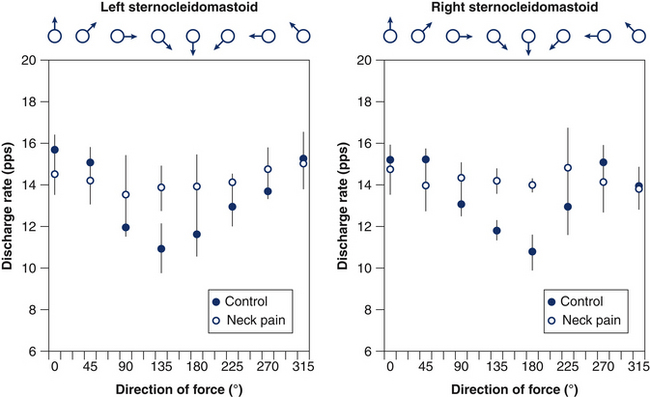
A recent study showed that when women with either whiplash-induced or idiopathic neck pain performed isometric circular contractions of their neck at a fixed force level but with changes in force direction in the range 0–360° (Figs 6.3a, b), they displayed reduced specificity of sternocleidomastoid muscle activation with respect to asymptomatic individuals.42 In particular, increased activation of the sternocleidomastoid muscle was evident when the muscle acted as an antagonist (Figs 6.3c, d). This observation indicates that the augmented activity of the superficial muscles in patients with neck pain reflects a loss of the predefined patterns of muscle activation and supports the consistent finding of augmented activity of the sternocleidomastoid muscle in such patients, regardless of the task examined; for example, cervical flexion,43 craniocervical flexion39, 44 and movements of the upper limb.40, 45
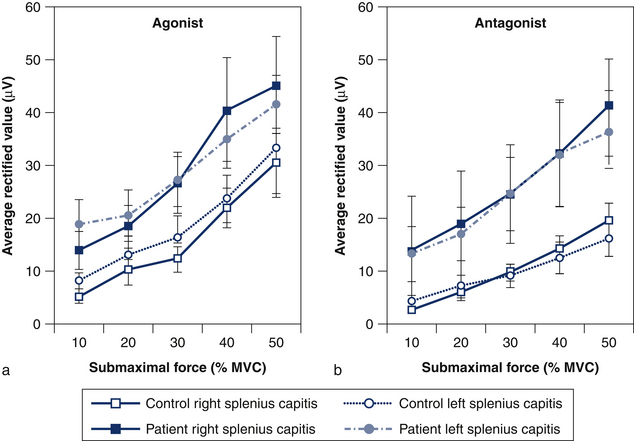
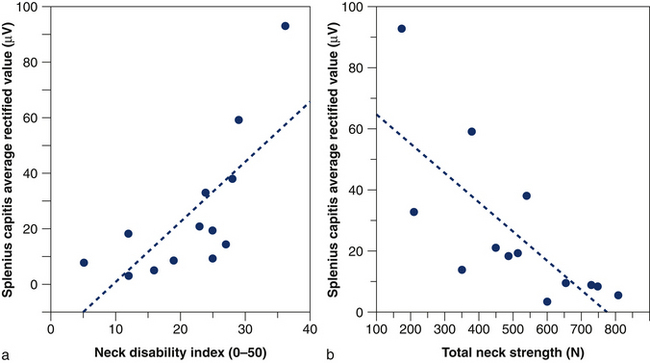
Reduced specificity of sternocleidomastoid muscle activity in patients with whiplash is associated with impaired neck flexion strength, which suggests that reduced strength is partially due to increased coactivation of their neck muscles.32 Likewise, increased coactivation of the splenius capitis muscle during isometric cervical flexion (Fig 6.4) is inversely correlated with total neck strength and positively correlated with the patient’s reported pain and perceived disability (Fig 6.5).32 The strategy adopted by the patients, despite being inefficient, may reflect an attempt to voluntarily increase the stability of the head for the fear of performing potentially painful movements or the need to compensate for decreased activity of the deep cervical muscles.39
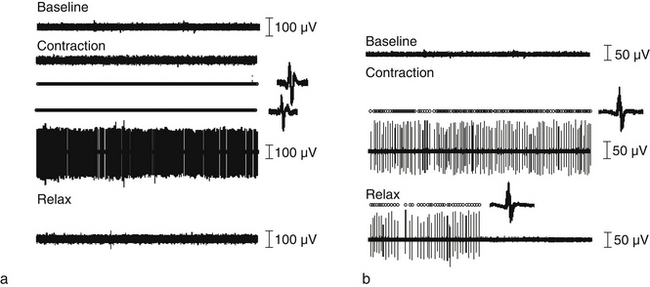
Direct recordings of single motor unit activity have been performed in order to better understand the mechanisms underlying augmented superficial neck muscle activity associated with neck pain. A recent study investigated the discharge rate of sternocleidomastoid motor units during brief isometric contractions performed in multiple directions of force in the horizontal plane.42 The discharge rates of active motor units during a task will depend on the net excitatory input to the motor neuron pool and the intrinsic excitability of the motor neurons. Thus, it is expected that the excitatory input changes with direction of force with a consistent modulation that reflects the biomechanical efficiency in force production. Observations of motor unit behaviour in asymptomatic individuals confirm this hypothesis by revealing a consistent modulation in discharge of motor units depending on the direction of force (Fig 6.6). In contrast, women with neck pain display the same neural drive to the sternocleidomastoid muscle regardless of the direction of force. This result additionally explains the consistent finding of increased activity of this muscle in patients with neck pain. Furthermore, the patient group, but not controls, showed prolonged motor unit activity in their neck muscles following a contraction (Fig 6.7),42 which confirms the reduced ability to relax muscles previously shown in surface EMG studies.45, 46 Taken together, reduced modulation of neural drive to the muscle and prolonged motor unit activity following a contraction may indicate a potential change in motor neuron excitability due to pain and/or sympathetic activity (discussed below).
In addition to their role in neck movement and stability, the neck muscles are involved in proprioception and reflexes controlling postural orientation and stability.47–49 Not surprisingly, whiplash-induced pain is also associated with impaired proprioception, reduced postural stability and reduced smoothness of eye movements. Alterations in proprioception may reflect abnormal spindle afferent discharge due to activation of chemoreceptive and nociceptive sensory afferent input,50–53 direct trauma to cervical structures, or increased sympathetic drive,54 resulting in a conflict of inputs from visual, vestibular and somatosensory sources; this may underlie the sensation of unsteadiness and dizziness frequently reported in whiplash patients.55 Although greater errors in positioning the head following voluntary movement have been shown in persons with neck pain of both insidious56 and traumatic onset,57–59 proprioceptive acuity appears to be more affected in people with chronic whiplash,58 especially in those reporting higher pain and disability36, 60 and dizziness.55 In addition to greater repositioning errors with head movement, people with whiplash show reduced proprioception at the shoulder and elbow.61, 62
Disturbed postural stability is also a feature of whiplash,63–65 which includes larger postural trunk sway in both stance tasks and complex tasks, such as walking up and down stairs,64, 66, 67 and reduced head stability in response to predictable and unpredictable postural perturbations.64 Although balance disturbances have been reported in both traumatic and non-traumatic neck pain disorders, consistent with observations for head relocation, people with whiplash-induced pain generally show greater disturbances,68 and postural instability is positively associated with the symptom of dizziness.69 Disturbances of postural control are also thought to reflect disruption of cervical somatosensory input and a mismatch between somatosensory, visual and vestibular inputs. Consistent with this hypothesis, changes in oculomotor control have also been demonstrated in people with whiplash, which is suggestive of disturbances in the cervicocollic and cervico-ocular reflexes.70–73
Nociceptive effects on sensorimotor function
Group III and IV muscle afferent receptors are sensitive to nociceptive stimuli74 and this can be experimentally elicited in humans using various exogenous or endogenous methods, including intramuscular injection of hypertonic saline.75 Experimental neck pain models provide a means to increase our understanding of the basic mechanisms underlying alterations of muscle activity in people with whiplash since injection of hypertonic saline produces a deep pain similar in quality to clinical pain.76
At rest, only a small and transient increase in postural electromyographic (EMG) activity has been observed in response to deep noxious stimulation of cervical muscles.77, 78 However, experimental pain research has demonstrated convincingly that nociception substantially alters the load sharing among muscles during activity, which may lead to subsequent muscle overuse or disuse in functional activities. Evidence for an altered motor strategy has been provided at levels ranging from the motor unit79 and motor unit pool80 to a reorganisation of activity among agonist, synergist and antagonist muscles,81, 82 and is known to occur immediately after the onset of pain.
As an example, hypertonic saline-evoked unilateral upper trapezius muscle pain induces an immediate bilateral reorganisation in the coordinated activity of the three subdivisions of the trapezius muscle during a dynamic task of the upper limb.82 Overall, the results of experimental studies suggest that pain induces a dynamic reorganisation of the coordination among muscles in order to minimise the use of the painful muscle with minimal disruption to the task. The reorganisation of motor strategy may be mediated by spinal (reflex) and supraspinal (cortical motor drive) mechanisms.
Although a reorganisation of neck muscle activity may have benefits in the short-term for reducing the load on the painful region, in the long term, pain-induced altered neural control may dispose some muscles to overload and, as a consequence, injury to other muscles due to reduced activity, with consequent atrophy of specific fibre types. Thus, long-term alterations in motor control may partially explain the morphological and histological changes documented in the cervical muscles in people with neck pain.83
Experimental studies also show that activation of nociceptive afferent fibres from neck muscles and joints affects the proprioceptive activity of muscle spindle afferent fibres50–52 which may partially explain impaired proprioception in patients with whiplash. Furthermore, preliminary findings suggest that activation of the sympathetic nervous system via the cold pressor test is sufficient to induce altered neck proprioception (unpublished observations).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree