In this review, we discuss the literature regarding both concussion and mild traumatic brain injury. We focus on the role for neuroimaging in the concussed patient and describe the recommended practices related to imaging in concussion. This discussion first focuses on the exclusion of severe injuries and is followed by a discussion of the potential utility of various advanced imaging techniques in research and clinical practice.
Key points
- •
There is a strong need to develop objective measures to ensure accurate and timely diagnosis of concussion and mild traumatic brain injury (mTBI) and to guide subsequent management decisions. Neuroimaging is likely to play an important role in this process.
- •
Despite the superior soft tissue contrast available by MRI, computed tomography (CT) remains the first-line imaging modality of choice in the acute setting due to its speed, ubiquitous availability, lower cost, and infrequent contraindications precluding the need for screening procedures.
- •
Although microstructural sequelae of concussion/mTBI are mostly below the threshold for standard CT and conventional MRI techniques, advanced MRI techniques (diffusion tensor imaging, functional MRI, perfusion, spectroscopy) and PET provide insight into these injuries.
Introduction
The phenomenon of concussion has received increasing attention in recent years primarily due to increased awareness of sports-related concussion (SRC) in adolescent and professional athletes, and in military personnel. The incidence of SRC is estimated at 1.6 to 3.8 million annually, and during the wars in Iraq and Afghanistan, up to 25,000 mild traumatic brain injuries (mTBIs) were reported each year in the US Armed forces. It is estimated that direct medical costs and indirect costs, such as lost productivity related to concussion and mTBI, total $12 billion per year in the United States alone. This increased attention and the substantial societal costs have led to the recent publication of new or updated practice guidelines and position statements from multiple medical and professional societies addressing the prevention, diagnosis, and management of concussion. The variations in these guidelines make clear that there is still a need to develop objective measures to ensure accurate and timely diagnosis of concussion and to guide subsequent management decisions. Neuroimaging is likely to play an important role in this process.
Concussion and mTBI labels are often used interchangeably; however, they should be considered distinct entities, at least for now. According to the 4th International Conference on Concussion in Sport, concussion is defined as the syndrome resulting from low-velocity injuries to the head that result in clinical symptoms but do not demonstrate visible structural abnormalities on conventional neuroimaging studies. Symptoms, which include headaches, dizziness, blurry vision, and difficulty concentrating typically, demonstrate rapid onset and are relatively short lived. mTBI is characterized by greater clinical symptoms and demonstrates some evidence of structural injury on conventional neuroimaging studies, but without the degree and duration of symptoms that would qualify as moderate TBI. Thus, concussion and mTBI occupy adjacent positions on the spectrum of TBI, with similar and overlapping clinical symptoms and distinguished by the presence or absence of findings on conventional neuroimaging. How long this differentiation will last is uncertain as more sensitive MRI techniques and higher magnet field strengths become available for clinical use, allowing for the detection of subtle injuries not visible with older techniques. As such, in this review we discuss the literature regarding both concussion and mTBI.
When athletes or military personnel experience concussive symptoms, there are 2 major questions that need to be answered acutely. The question of most immediate importance is whether there is an associated structural abnormality, such as intracranial hemorrhage or fracture, which may need immediate intervention. These are typically detected by computed tomography (CT). The second major question to be addressed is what is the appropriate time to return to play or return to active duty. This decision is not trivial given that were a new head injury to occur before the symptoms of the previous injury have resolved, there is increased risk of severe brain injury and potentially death, even in the setting of a relatively mild trauma. This is known as “second impact syndrome.” Currently, the decision to return to the field is guided by symptoms and various sideline assessment tools, but there is potential for neuroimaging techniques to help guide these decisions, both acutely and in the long term.
In this review, we focus on the role for neuroimaging in the concussed patient and describe the recommended practices related to imaging in concussion. This discussion first focuses on the exclusion of severe injuries and is followed by a discussion of the potential utility of various advanced imaging techniques in research and clinical practice.
Introduction
The phenomenon of concussion has received increasing attention in recent years primarily due to increased awareness of sports-related concussion (SRC) in adolescent and professional athletes, and in military personnel. The incidence of SRC is estimated at 1.6 to 3.8 million annually, and during the wars in Iraq and Afghanistan, up to 25,000 mild traumatic brain injuries (mTBIs) were reported each year in the US Armed forces. It is estimated that direct medical costs and indirect costs, such as lost productivity related to concussion and mTBI, total $12 billion per year in the United States alone. This increased attention and the substantial societal costs have led to the recent publication of new or updated practice guidelines and position statements from multiple medical and professional societies addressing the prevention, diagnosis, and management of concussion. The variations in these guidelines make clear that there is still a need to develop objective measures to ensure accurate and timely diagnosis of concussion and to guide subsequent management decisions. Neuroimaging is likely to play an important role in this process.
Concussion and mTBI labels are often used interchangeably; however, they should be considered distinct entities, at least for now. According to the 4th International Conference on Concussion in Sport, concussion is defined as the syndrome resulting from low-velocity injuries to the head that result in clinical symptoms but do not demonstrate visible structural abnormalities on conventional neuroimaging studies. Symptoms, which include headaches, dizziness, blurry vision, and difficulty concentrating typically, demonstrate rapid onset and are relatively short lived. mTBI is characterized by greater clinical symptoms and demonstrates some evidence of structural injury on conventional neuroimaging studies, but without the degree and duration of symptoms that would qualify as moderate TBI. Thus, concussion and mTBI occupy adjacent positions on the spectrum of TBI, with similar and overlapping clinical symptoms and distinguished by the presence or absence of findings on conventional neuroimaging. How long this differentiation will last is uncertain as more sensitive MRI techniques and higher magnet field strengths become available for clinical use, allowing for the detection of subtle injuries not visible with older techniques. As such, in this review we discuss the literature regarding both concussion and mTBI.
When athletes or military personnel experience concussive symptoms, there are 2 major questions that need to be answered acutely. The question of most immediate importance is whether there is an associated structural abnormality, such as intracranial hemorrhage or fracture, which may need immediate intervention. These are typically detected by computed tomography (CT). The second major question to be addressed is what is the appropriate time to return to play or return to active duty. This decision is not trivial given that were a new head injury to occur before the symptoms of the previous injury have resolved, there is increased risk of severe brain injury and potentially death, even in the setting of a relatively mild trauma. This is known as “second impact syndrome.” Currently, the decision to return to the field is guided by symptoms and various sideline assessment tools, but there is potential for neuroimaging techniques to help guide these decisions, both acutely and in the long term.
In this review, we focus on the role for neuroimaging in the concussed patient and describe the recommended practices related to imaging in concussion. This discussion first focuses on the exclusion of severe injuries and is followed by a discussion of the potential utility of various advanced imaging techniques in research and clinical practice.
Deciding when/if to image the concussed patient
Consensus recommendations from the 4th International Conference on Concussion in Sport, the American Medical Society for Sports Medicine, the American Academy of Neurology Concussion Guidelines, and the American College of Emergency Medicine/Centers for Disease Control and Prevention joint practice guidelines recommend that head CT be performed for individuals with more than 30 seconds of loss of consciousness, prolonged altered mental status, severe headache, focal neurologic deficits, or seizure. There is, however, substantial overlap in the clinical symptoms of patients both with and without radiologically evident acute traumatic injuries on CT, and patients may be found to have acute intracranial findings on head CT in the setting of an unimpressive examination. An additional consideration is the radiation dose from imaging. Most relevant is the stochastic effect, which is the likelihood of radiation exposure inducing cancer. Routine CT of the brain has an effective dose of 2 mSv and 1 CT brain scan is estimated to increase the lifetime risk of cancer by 0.016% in men and 0.026% in women. As such, is it ultimately up to the clinical judgment of the caregiver whether or not to proceed to imaging, weighing the benefits of imaging against the costs, including financial costs and radiation exposure.
Structural imaging: excluding acute traumatic brain injuries
In the past, conventional plain film radiography of the skull had a role in the diagnosis of skull fractures after head injury; however, this has been essentially supplanted by CT. Despite the superior soft tissue contrast available by MRI, CT remains the first-line imaging modality of choice due to its speed, ubiquitous availability, lower cost, and infrequent contraindications precluding the need for screening procedures.
Computed Tomography
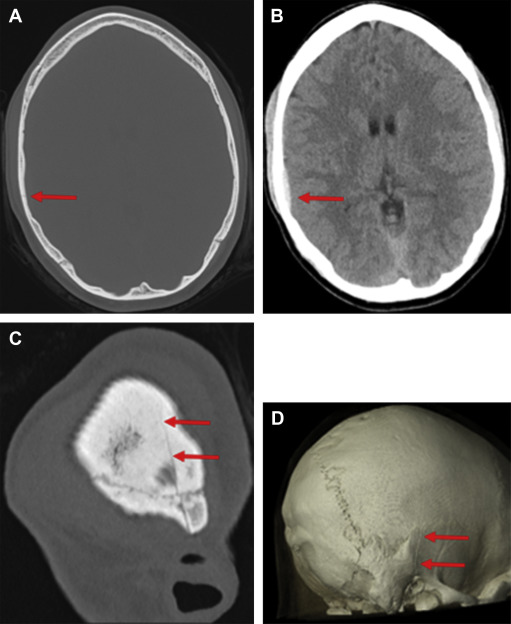
CT scanning uses a rotating x-ray tube and rotating x-ray detectors that measure x-ray attenuation and process detector data into gray-scale images. CT is highly sensitive for the detection of calvarial and facial fractures, as well as hemorrhage in the epidural, subdural, subarachnoid, intraventricular, and parenchymal spaces. It is also moderately sensitive to cerebral contusions that may or may not require neurosurgical intervention. The precise incidence of traumatic intracranial injuries in the setting of SRC is not available, but there are some data on patients with mTBI of all causes. The literature shows variable rates of injury detected on CT, between 16% and 21% in one study of 912 patients with mTBI, but as low as 3% and as high as 34% in other studies. Acute injuries necessitating neurosurgical intervention, however, are low across studies, approximately 1% or less. The sensitivity for detecting both skull fractures and subtle intraparenchymal and extra-axial hemorrhages can be improved by thin collimation acquisition using both 2-dimensional (2D) multiplanar and 3D reconstructions ( Fig. 1 ).
Magnetic Resonance Imaging
MRI also has a role in imaging selected patients with suspected head trauma in the acute setting. As opposed to CT, MRI does not depend on ionizing radiation, but instead uses a strong magnetic field and radiofrequency pulses to excite spinning protons in tissues to create imaging with numerous different tissue contrasts. Given its higher cost and other limitations, MRI is typically reserved for patients in whom the initial CT was deemed negative but whose symptoms are not improving or to further evaluate patients in whom the initial CT demonstrated brain injury.
MRI outperforms CT for the detection of parenchymal injuries, including diffuse axonal shear injury (DAI) and small parenchymal contusions ( Fig. 2 ). MRI is more sensitive than CT in the detection of subdural and epidural hematomas, whereas CT is more sensitive to subarachnoid hemorrhage. Fluid attenuated inversion recovery (FLAIR) imaging is particularly sensitive to parenchymal edema, allowing identification of small contusions that can be missed on CT. There is also excellent conspicuity of extra-axial hematomas on FLAIR MRI, allowing for the detection of thin hematomas, which can be inconspicuous on CT ( Fig. 3 ). T2 star (T2*)-weighted gradient-echo imaging (GRE) exploits the distortions in magnetic fields created by paramagnetic material, which makes it exquisitely sensitive to blood products in certain stages of degradation. GRE is therefore very sensitive to the microhemorrhages associated with DAI. In patients with neurologic changes in the setting of rapid deceleration injury, for example, MRI should be performed, as DAI is frequently missed on CT ( Fig. 4 ). Susceptibility-weighted imaging (SWI), a newer iteration using the susceptibility principle, further increases sensitivity for susceptibility-related signal, resulting in greater susceptibility signal blooming and increased lesion conspicuity. Although MRI is more sensitive for small lesions, severe injuries necessitating neurosurgical intervention are typically easily detected by either CT or MRI.
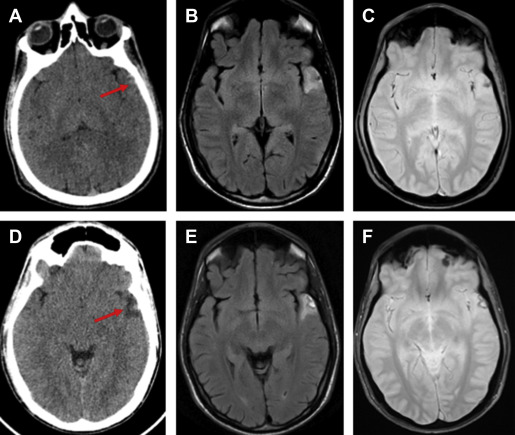
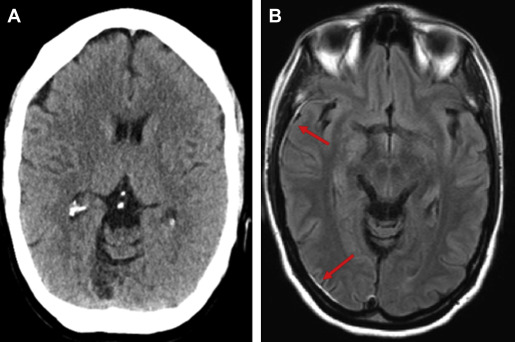
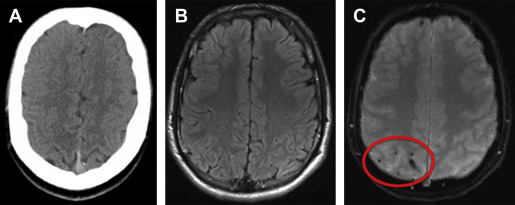
The clinical utility of MRI for the detection of small areas of brain injury not visible on CT has recently been investigated. Yuh and colleagues, in a multicenter prospective study of 135 consecutive patients with mTBI in the emergency department evaluated for acute head injury demonstrated that small parenchymal contusions or 4 or more sites of hemorrhagic shearing detected by MRI but not visible on CT were independent predictors of poorer 3-month outcome as measured by the Extended Glasgow Outcome Scale at 3 months after injury.
Advanced and experimental imaging techniques for the evaluation of concussion
Concussion is generally believed to be a functional, rather than structural, brain injury, which develops when trauma to the head places the brain under translational and/or rotational acceleration-deceleration forces. These forces result in injuries to cell membranes and ionic pumps, causing pathologic ionic cascades and loss of neurochemical homeostasis, with resulting clinical symptoms. Although microstructural sequelae of most of these traumas are below the threshold for standard CT and conventional MRI techniques, more advanced MR techniques are being developed to assess the full extent of injury. These techniques include ultra-high field structural imaging, diffusion tensor imaging (DTI), functional MRI (fMRI), perfusion imaging, PET scanning, and magnetic resonance spectroscopy (MRS).
Ultra-High Field Structural Imaging
MRI is typically performed with 1.5 T (1.5 T) or 3.0 T magnetic field strengths and conventional MRI sequences including GRE, SWI, and FLAIR show improved sensitivity at 3.0 T versus 1.5 T. Ultra-high field MRI at field strengths of 7 T and beyond is being investigated for use in detection of microstructural traumatic injuries, although this is not in widespread clinical use. One study assessing the performance of SWI at 7 T and 3 T in patients with mTBI found that at 7 T up to 41% more microhemorrhagic shearing injuries were identified than at 3 T, and the shearing injuries visible at 3 T were shown to be significantly larger at 7 T. The 7-T MRI may also improve tissue specificity, with one preliminary study showing that in some cases of concussion, suspected shearing injuries visible at 3 T were found to be developmental venous anomalies at 7 T. The greatly increased detection of microstructural injury in the setting of mTBI may eventually lead to a redefinition of concussion as a microstructural traumatic derangement, rather than a purely “functional” phenomenon, although this has yet to be demonstrated.
Diffusion Tensor Imaging
Diffusion-weighted imaging (DWI) is a specialized imaging sequence that images the Brownian motion of water molecules as they diffuse through the extracellular space, irrespective of the direction of diffusion. DTI builds on DWI by characterizing the directionality of the diffusion of water in 3D space by measuring the diffusion in at least 6 different directions, but potentially up to hundreds. Given that the diffusion of water is highly influenced by tissue microstructure, DTI is especially useful for measuring the microstructural integrity of highly ordered tissues, such as the white matter, via fractional anisotropy (FA). FA is represented as a value between 0 and 1, in which 0 indicates a completely isotropic environment in which there is no restriction to diffusion in any direction, and 1, in which water is capable of diffusing in only 1 axis. White matter in the commissures, such as the corpus callosum, typically have FA values between 0.6 and 0.8, but FA in the rest of the white matter is typically lower ( Fig. 5 ). Besides FA, other DTI parameters can be evaluated, including mean diffusivity (MD), which is overall magnitude of the diffusivity; radial diffusivity (RD), which is the diffusivity perpendicular to a fiber bundle; and axial diffusivity (AD), which is the diffusivity along the fiber bundle. There has been some suggestion that RD may be more specific to myelin damage, and AD more specific to axonal damage, but this is probably a convenient generalization made in the early stage of a rapidly developing field.
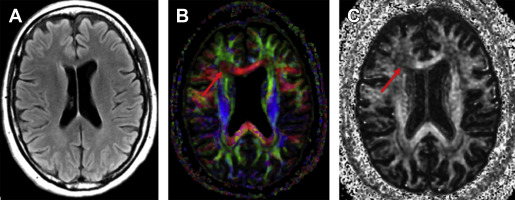
DTI may be a valuable tool for assessment of patients with concussion and mTBI. Numerous studies have described changes in various DTI metrics following concussion and TBI, typically in patients in the subacute or later stages from the initial injury and with persistent symptoms. The results of these studies vary, with many showing reduced FA or increased MD, indicative of microstructural damage in concussion and patients with mTBI compared with controls; however, other studies show the opposite findings, with elevated FA or decreased MD, whereas still others show mixed or no differences. Microstructural abnormalities in these studies are found widely throughout the brain, including the anterior and posterior corpus callosum, internal capsules, frontal and parietal corona radiata, and the uncinate fasciculus. Some studies also showed that alterations in diffusivity correlate with the severity of postconcussive symptoms. These studies demonstrate group differences in DTI parameters, making their applicability in the individual patient of little use. There are ongoing efforts to assess the potential of DTI for prognostic purposes in individual patients. Yuh and colleagues, in a recent study of 76 patients with mTBI, demonstrated reduced FA compared with control subjects and found that reduced FA was a significant predictor of both 3-month and 6-month outcomes measured by the Glasgow Outcome Scale. More work is needed before a consensus can be reached regarding the best way to use DTI for diagnosis and prognosis in individual patients.
Functional MRI
fMRI relies on the coupling of increased neuronal activity and compensatory changes in vascular perfusion to create maps of cerebral activity. When neurons activate, there is increased extraction of oxygen from local tissues, which triggers an overcompensating vasodilatory response that results in elevated levels of oxyhemoglobin and relatively decreased level of deoxyhemoglobin. This relative decrease in the amount of deoxyhemoglobin is measured through blood oxygen level–dependent (BOLD) imaging. BOLD fMRI can be performed either with task-based paradigms, or in the resting state. During task-based BOLD fMRI, patients are imaged during alternating periods of task performance or stimulus presentation, and rest. Therefore, the differences in BOLD signal between the active and passive phases appear as regional activations associated with the task or stimulus. For resting state BOLD fMRI, subjects are imaged entirely at rest and the temporal relationships of spontaneous low-frequency variations in BOLD signal are used to assess the functional connectivity of various brain regions.
Numerous studies have investigated patients with concussion and patients with mTBI with task-based fMRI. Most studies have used tasks that focus on working and declarative memory, attention, and other aspects of executive functioning. Studies comparing symptomatic, concussed adult patients and healthy controls commonly demonstrate little or no differences in performance of functional tasks. On fMRI, adults with concussion demonstrate either abnormal hypo-activation or hyper-activation in regions related to executive functioning, such as the dorsolateral prefrontal cortex (DLPFC) and parietal lobes, as well as increased activation in more widely distributed brain regions, suggesting recruitment of these areas. These functional abnormalities may persist for several months but may eventually normalize. This suggests that for a concussed individual to satisfactorily perform a given task, his or her brain may have to work harder, by engaging compensatory strategies that include increased alterations in activity in task-related regions and/or recruitment of more widespread brain regions than healthy controls. The picture in adolescents may be different. Keightley and colleagues assessed 15 adolescents with concussion and 15 matched controls using BOLD fMRI and a standardized working memory task. In contradistinction to adults, concussed adolescents performed poorer on the task than controls, with reduced task related performance in the bilateral DLPFC, left premotor cortex, left superior parietal lobule, and other widely distributed regions. Interestingly, task performance was significantly correlated with signal changes in the DLPFC (greater DLPFC activation was associated with greater accuracy on the task). This finding, in the setting of reduced task performance and reduced activation in the DLPFC, suggests that unlike adults, concussed youths may be unable to adequately compensate to maintain cognitive performance. This may have implications in “return-to-play” decisions.
Resting state fMRI studies examining functional connectivity demonstrate decreased or increased connectivity between various brain regions after concussion. Mayer and colleagues have shown decreased functional connectivity within the default mode network (DMN) and hyperconnectivity between the DMN and lateral prefrontal cortex, and others have found both reduced connectivity in posterior regions of the DMN (posterior cingulate, parietal lobe) and increased connectivity in the anterior portions of the DMN (medial prefrontal cortex). Abnormalities in numerous other functional networks, including those involved in cognitive and motor control and visual processing, also have been reported. Obviously, findings are varied/mixed and limited conclusions can be drawn regarding the consistency and significance of these data, as most studies at this point have been limited by relatively small sample sizes and varying time points from injury.
Ultimately, both task-based and resting state fMRI have demonstrated group differences between patients with concussion/mTBI and controls. Although there is variation in the results, the major themes implicate dysfunction in executive functioning, particularly in the frontal lobes; youths are more likely to develop cognitive deficits than are adults; and that concussed subjects who perform comparably to controls may be able to compensate for the injury through recruitment of other brain regions supporting the given task.
Perfusion Imaging
Several studies using perfusion-imaging techniques have been performed in recent years to assess changes in regional blood flow, a surrogate for metabolism, in the brain in patients with mTBI and normal conventional brain imaging findings. These studies have demonstrated regional perfusion deficits that have correlated with posttraumatic cognitive and psychological impairment. A variety of imaging techniques have been used in this regard, including perfusion CT, dynamic susceptibility contrast, and arterial spin-labeling (ASL) MR perfusion imaging, single-photon emission computed tomography (SPECT), and PET. No single modality has emerged as singularly more useful than the others in the evaluation of mTBI; the relative advantages and disadvantages of each modality are therefore discussed.
Perfusion computed tomography
Perfusion CT is performed by continuously scanning through the brain during the first pass of an intravenous contrast bolus. This yields time-density curves for each voxel from which cerebral blood volume (CBV), cerebral blood flow (CBF), mean transit time, and time to peak can be calculated. Postprocessing software generates a complete set of parametric maps typically within a few minutes of completing a scan.
In current clinical practice, perfusion CT is not routinely used in the evaluation of TBI. Perfusion CT is obtained in acute TBI, when there is suspected concurrent vascular injury and acute ischemic infarct. A few studies have investigated the utility of perfusion CT specifically for assessment of TBI, demonstrating a higher sensitivity for the diagnosis of cerebral contusions when compared with noncontrast CT at the time of hospital admission, with reported sensitivity of 87.5% versus 39.6%. Additionally, these scans obtained in the acute phase were independently predictive of functional outcome at 3 to 6 months, with normal brain perfusion or hyperemia predicting favorable outcomes and oligemic patterns predictive of unfavorable outcomes. The examination requires substantial radiation, however, and appropriate selection of patients who would benefit most from this examination is therefore critical. Further studies will be required before the question of appropriateness is resolved.
Perfusion MRI
MRI is an appealing modality for assessment of perfusion abnormalities in mTBI/concussion given the lack of ionizing radiation, typically performed whether with dynamic susceptibility contrast (DSC) or arterial spin labeling (ASL). DSC is performed by measuring signal change during the first pass of an intravenous bolus of gadolinium-based contrast, and using the tissue concentration–time curves to generate maps of relative CBF and volume (rCBF and rCBV, respectively). ASL labels flowing blood with radiofrequency pulses creating an “endogenous contrast” that can be used to calculate CBF. ASL does not require an intravenous contrast agent, and is therefore completely noninvasive and repeatable. In addition, truly quantitative values of CBF can be obtained rather than relative values. DSC, however, provides a higher signal-to-noise ratio and higher spatial resolution compared with ASL.
Only a few small studies have explored the utility of perfusion MRI for evaluation of mTBI with imaging primarily performed in the subacute to chronic phases of injury. Both DSC and ASL were able to detect reductions in relative CBF and CBVs within in various regions of the brain, which correlated with associated neurocognitive deficits. Further research is required to determine the utility of these examinations in this setting.
Single-photon emission computed tomography
SPECT imaging involves injection of a radiopharmaceutical into the bloodstream, which then accumulates in regions of the body according to its biological properties. The most commonly used radiopharmaceuticals for SPECT imaging of the brain are technetium-99m-hexamethylpropyleneamine oxime (99mTc-HMPAO) and 99m Tc-ethyl cysteinate dimer. These radiotracers travel to regions of the brain proportional to perfusion and are taken up by brain cells.
Studies have shown SPECT perfusion imaging to be sensitive to mTBI, showing decreases in CBF to various areas of the brain and SPECT may be more sensitive compared with conventional CT or MRI in both the acute and chronic settings. Furthermore, SPECT lesion localizations have been shown to be concordant with neuropsychological tests. SPECT has a strong negative predictive value, with TBI-associated symptoms resolving within 3 months in 89% to 97% of patients with negative SPECT examination within the first month following injury and 100% negative predictive value when performed at 1 year after injury. Positive predictive value of SPECT increases over time with 59% of those with an abnormal SPECT in the first month after injury remaining symptomatic at 3-month follow-up, compared with 83% when imaged at 1 year after injury. Although these results are promising, current limitations in the evidence to support the routine clinical use of SPECT perfusion imaging in the evaluation of mTBI include very few randomized controlled trials and various levels of analytical rigor used to identify TBI-associated changes with SPECT imaging.
Positron Emission Tomography
PET is an imaging method that requires injection of a radiopharmaceutical, followed by scanning performed at a set interval. A wide variety of PET radiopharmaceuticals are available for brain studies, some of which include those used to image cerebral perfusion; glucose, protein, and oxygen metabolism; amyloid deposition; and neurotransmitter systems. PET has higher spatial resolution than SPECT and suffers from fewer artifacts, albeit at a higher cost.
Much of the research evaluating mTBI with PET has focused on identifying metabolic anomalies using 18F-Fluorodeoxyglucose (18-FDG). Global and focal alterations in glucose metabolism have been observed after mTBI. A few small studies in the setting of chronic mTBI have shown correlation between findings on 18-FDG images and cognitive tests ; however, data should be considered preliminary and the literature is as yet inconclusive.
A recently developed compound, [18F]-fluoroethyl-methyl-amino-2-naphthylethylidene-malononitrile (FDDNP), has affinity for pathologic deposits of beta-amyloid and tau protein, both of which are found in various neurodegenerative diseases. This compound may potentially serve as a marker for chronic traumatic encephalopathy (CTE), which is believed to be a progressive tauopathy, found in individuals who have had repetitive concussive brain injuries. Currently, there is no definitive clinical diagnosis of CTE, and as such it would benefit from a method of early diagnosis, particularly for the development of experimental therapeutic interventions. Preliminary investigations have demonstrated promise for this radiopharmaceutical while research is currently ongoing. Another newer compound, [18F]-THK523, shows promise as a tau-specific marker.
Magnetic Resonance Spectroscopy
Metabolites may be identified by their chemical shift resonance in MRI, expressed in parts per million (ppm), independent of the field strength used. In the brain, N-acetyl aspartate (NAA, at 2.01 ppm) is a marker of neuronal population and function; total choline (Cho, at 3.2 ppm), including primarily phosphoryl and glycerophosphoryl choline, reflects turnover of membranes; creatine (Cr, at 3.0 ppm), composed of phosphocreatine, is considered a reference value related to energetic metabolism; myo-inositol (mI, at 3.56 ppm) is related to glial proliferation; glutamate/glutamine complex (Glx, at 2.12–2.35 and 3.74–3.75 ppm) is the major excitatory neurotransmitter in the brain; and lactate (Lac, at 1.33 ppm) is related to anaerobic glycolysis. It is important to note that the levels of metabolites vary with the anatomic location evaluated and with aging.
As a noninvasive method capable of revealing the metabolic profile in the brain, MRS may be a suitable method for both initial diagnosis and longitudinal follow-up in SRC; however, this utility currently remains essentially restricted to research. Various combinations of metabolites identified by single-voxel or multivoxel techniques may potentially reveal the neurometabolic imbalance of inflammatory and excitatory cascade metabolites related to the pathophysiology of trauma. In the acute setting of SRC (1–6 days), decreased NAA has been demonstrated in the frontal lobes, which was correlated with self-reported symptoms and/or neuropsychological testing results, with variable deficits in attention, verbal memory, visual memory, information-processing speed, and reaction time. Decreased Cr and mI levels in the DLPFC and decreased Glx in the primary motor (M1) region have been found in nondiagnosed asymptomatic collision high school football athletes over the season, reflecting ongoing metabolic disturbance despite lack of clinically reportable symptoms.
Decreased NAA/Cr and NAA/Cho ratios are believed to reflect impaired energetic metabolism, occurring during the post concussive vulnerable period of the brain. This window of vulnerability to a second minor trauma leading to second-impact syndrome has been demonstrated by MRS. Three days after concussion, decreased NAA/Cr and NAA/Cho ratios in bilateral frontal white matter were shown to return to normal after 30 days in athletes who suspended practice, as opposed to after 45 days in athletes who had a second concussion within 15 days of the first one. Reported resolution of symptoms was between 3 and 15 days for the patients with a single concussion and at 30 days for the patients with repeat concussion, earlier than the resolution of MRS changes. Variability in the levels of Cr, originally thought to be constant in the brain, has also been demonstrated on longitudinal SRC studies, perhaps reflecting decreased energy metabolism levels seen in more severe cases. Demonstration of increased mI/Cr and Glx/Cr levels in the M1 region in concussed football athletes after 6 months of injury, not present in the acute phase, suggests metabolic derangement as a dynamic process that may evolve over time.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




