Fig. 8.1
Scheme depicting the two major mechanisms purported to underlie the development of muscle cramps
8.5.1 Central Origin Theory
Norris et al. (1957) proposed the central origin theory after performing experiments involving electromyography (EMG). Their study showed that synchronized discharges between different motor units were observed in cramping muscle. Moreover, voluntary contraction of the homologous muscle in the contralateral limb increased cramp discharge, while voluntary contraction of the ipsilateral antagonist muscle reduced cramp discharge. The authors concluded that the neural activity during muscle cramp originates from the spinal cord. Although this observation was anecdotal, the following studies have observed similar phenomenon in laboratory experiments.
Several studies have recorded motor unit activities during cramps (Minetto et al. 2009b, 2011; Norris et al. 1957; Ross and Thomas 1995). Shapes of the motor unit potentials (Ross and Thomas 1995; Minetto et al. 2011), and firing rates (Minetto et al. 2009b) during muscle cramps resemble those seen in normal voluntary contractions. However, the variability of motor unit firing rates was larger during cramp than during voluntary contractions (Minetto et al. 2009b, 2011; Ross and Thomas 1995). The high variability of motor unit discharge is speculated to be caused because the afferent inputs to the motor neurons generate synaptic noise (Merletti et al. 2011; Minetto et al. 2009b). However, since variability of motor unit discharge during cramp without afferent contribution by peripheral nerve block is greater than that during normal cramps (Minetto et al. 2011), the above mentioned explanation seems precarious. Thus more substantial evidence is required to implicate spinal involvement in cramp production.
Other studies have produced more direct evidence for spinal involvement in cramp production by investigating the spinal reflex (e.g. Mills et al. 1982; Baldissera et al. 1994; Khan and Burne 2007). In a case report Mills et al. (1982) noted that transcutaneous nerve stimulation over the calf muscle (by which afferent signals affect spinal neural activity) could abort cramps in a patient who suffered from severe muscle cramps. From this result they inferred that the mechanism of muscle cramps must involve the spinal cord. However, since only one patient was involved, the results cannot be generalized. Alternatively, recent studies report that nociceptive stimulation by a bolus injection of glutamate into latent myofacial trigger points induces cramps (Ge et al. 2008). In addition, increased nociceptive muscle afferent activity induced by injection of hypertonic saline decreased the threshold frequency of electrically elicited muscle cramps (Serrao et al. 2007). However, since these techniques may affect not only afferent activity but also the muscle itself, it does not conclusively indicate CNS involvement. Other studies have clarified the relationship between the kinesthetic afferent input and muscle cramps. Baldissera et al. (1994) showed that electrical stimulation to the Ia afferent nerve with low intensity triggered a cramp in the soleus muscle in three subjects who suffered from muscle cramps. In addition, the finding that taps and continuous vibration to the Achilles tendon also could induce the cramp (Baldissera et al. 1994) indicates that the afferent signals may also influence cramps. Moreover, Khan and Burne (2007) observed that EMG activity during cramp of calf muscle was inhibited by the electrical stimulation applied to the Achilles tendon. The intensity and timing of the disappearance of the EMG activity were similar to those of voluntary contractions seen in the same muscle at similar background EMG levels. Thus the authors suggested that the same reflex pathway was involved in the inhibition of both voluntary contractions and muscle cramps. Additionally, Ross and Thomas (1995) indicated that the tonic vibration reflex was depressed or absent after muscle cramps, while it never changed after voluntary contractions. This suggests that muscle cramps may inhibit the function of the spinal reflex. In contrast, prolonged enhancement of the H-reflex after cramping was observed, but it was not observed after voluntary contractions (Ross 1976). Because the H-reflex reflects the excitability of the motor neuron pool in the spinal cord, these two studies seems to be in conflict; one suggests inhibition and the other suggests the enhancement of the spinal neural activity. However, the fact that a muscle cramp has prolonged overall effects on neural excitability of the spinal cord is very likely.
Although many studies have proposed a central origin theory, the proposed mechanisms purporting to explain muscle cramps are different. Ideas put forth includes hyperexcitability of motor neurons with presynaptic inputs produced by a positive feedback loop between afferent nerves and motor neurons (Ross and Thomas 1995), bistability of the motor neuron membrane (Baldissera et al. 1994), and dysfunction of interneurons via GABA (Obi et al. 1993). When looked at closely, these ideas must be considered suppositions, since they are not supported by concrete evidences.
8.5.2 Peripheral Origin Theory
The first study that supported the peripheral origin theory utilized anesthesia to produce a peripheral nerve block (Lambert 1968). The nerve block shut off the contribution of supraspinal neural activity. Thus, efferent signals from the motor neuron pool could not reach the muscles, and afferent signals from sensory receptors were also unable to reach the spinal cord. It was thus possible to investigate the role of the nerves distal to the blocked point. Lambert (1968) observed that, in healthy subjects, repetitive electrical stimulation of the peripheral nerve distal to the block could induce a muscle cramp. This result strongly suggested that muscle cramps could occur without a supraspinal contribution, and cramps likely originate in the periphery, probably in the intramuscular nerve terminal. In later work, Bertolasi et al. (1993) replicated the induction of muscle cramps during a peripheral nerve block. In addition, they indicated that no muscle cramp was induced without a shortening of the muscle even when electrical stimulation was delivered, and also found that stretching to the muscle could interrupt cramps even after the nerve block in normal subjects. These data suggest that muscle length strongly influences muscle cramps, and that muscle cramps probably originate from the periphery, in particular intramuscular branches, rather than from the CNS. It seems that the peripheral origin theory was mainly based on the two above-mentioned studies.
Although the two studies (Bertolasi et al. 1993; Lambert 1968) appear to provide concrete evidences, other some studies have provided differing results. In 1993 Obi et al. showed that high-frequency electrical stimulation to the peripheral nerve distal to the blocked portion did not induce a muscle cramp. They additionally indicated that diazepam or baclofen, a GABA receptor agonist, prevented the induction of cramps by electrical stimulation. They speculated that abnormal activity of GABAergic interneurons in the spinal cord were involved in the mechanisms underlying cramps. However, this was an anecdotal report. There were no controls, and the study involved only two patients, both of whom had a motor neuron disease. A more convincing study was performed by Minetto et al. (2011), who provided evidence against the peripheral origin theory with experiments utilizing peripheral nerve block. They studied eight normal subjects, and were able to induce muscle cramps by electrically stimulating the muscle motor point with or without nerve block. They investigated the difference in characteristics of surface EMGs and motor unit potentials between cramps electrically induced under the two conditions. The results indicated that the threshold frequency of electrical stimulation for eliciting cramps was greater in the blocked condition than in the non-blocked condition. In addition, the duration and EMG amplitude of muscle cramps in the blocked condition were noticeably smaller. Cramps in the blocked condition showed a higher rate of motor unit discharge as well as irregular discharge patterns. The authors concluded that the CNS is involved in both the origin and sustenance of muscle cramps, rather than peripheral mechanisms.
In addition, Roeleveld et al. (2000) examined the detailed characteristics of muscle activity by multi-channel surface EMG recordings on the triceps surae during muscle cramps. They observed that involuntary EMG activity (i.e., muscle cramps) induced by maximal voluntary contraction (MVC) initially occurred over a small area and gradually spread over a larger region. Moreover, the strongly-activated area moved from one area to another, and then the intensity and area of the cramp decreased and disappeared. The results from these local EMG recordings indicate that muscle cramp might originate from close to, or even at, the level of the muscle fiber.
Recently, we investigated the involvement of peripheral mechanisms in muscle cramps in nine healthy subjects who were able to volitionally evoke muscle cramps of the abductor halluces (AH) by voluntary contraction (Nakagawa et al. 2013). High-intensity electrical stimulation to the tibial nerve, which induced a maximal M-wave (Mmax), was applied during the muscle cramp as well as during voluntary contraction of the abductor halluces. This was done to evaluate peripheral involvement, since the amplitude of Mmax is not affected by spinal activity. Subjects first voluntarily elicited a maximal voluntary contraction (MVC) of the target muscle in order to induce a cramp. Once the cramp occurred, the subjects were instructed to cease the volitional input and remained relaxed until the cramp diminished naturally. Throughout the trial, electrical stimulation to the tibial nerve was applied every 3 s, and the evoked EMG activity recorded. The onset of the cramp was defined as the moment when the subjects declared “I’m cramping” and the offset as the moment when the EMG burst stopped. The results indicated that the amplitude of Mmax decreased or disappeared during a muscle cramp, but not during voluntary contraction task (Fig. 8.2). We suspect that the decrease of Mmax during MVC that was seen before the occurrence of the cramp (as defined), happened during the time when the cramp had actually begun but before conscious awareness of this occurrence was attained by the subject. A significant negative correlation between Mmax amplitude and intensity of background EMG was observed during the muscle cramp task. The larger the background EMG, the greater the decrease in the amplitude of Mmax, although Mmax did not change during the voluntary contraction period (Fig. 8.3). Notably, the amplitude of Mmax in the soleus obtained simultaneously with the M-wave in the AH was not changed during the AH muscle cramp (Fig. 8.2). Overall, the results strongly suggest that the abnormal discharge seen during the muscle cramp occurred distal to the site of stimulation. However, this result does not directly exclude the possibility of spinal reflex involvement.
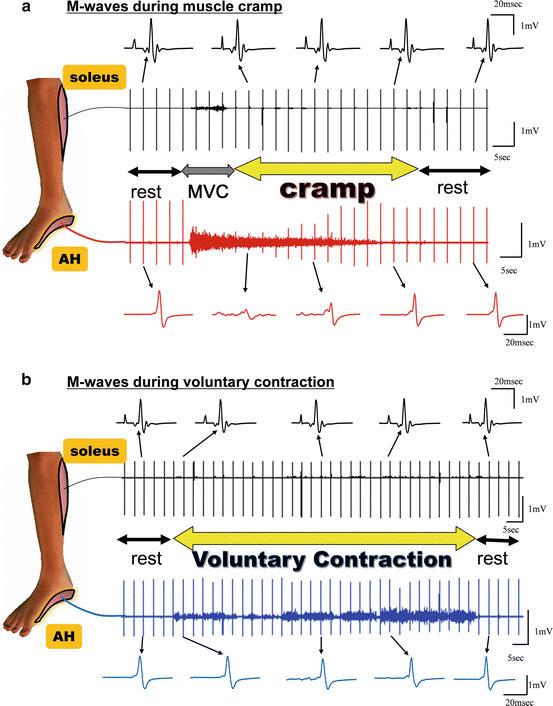

Fig. 8.2




(a) Modulation of maximal M-waves during muscle cramp and (b) voluntary contraction. The abductor hallucis (AH) is the cramping muscle, and the soleus is the control muscle that is not cramping. The onset of the cramp was defined as the moment when the subjects declared “I am cramping” and the offset as the moment when the EMG burst stopped
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








