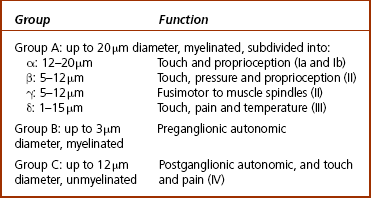
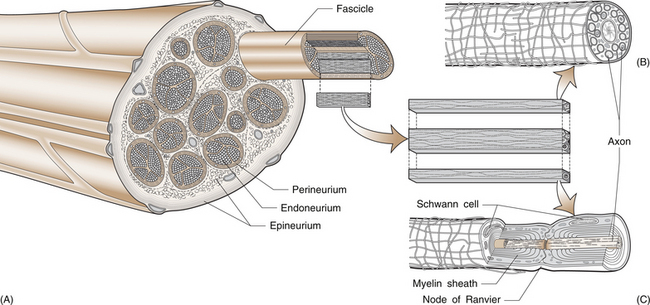
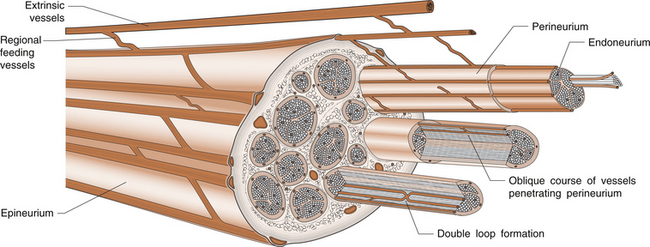
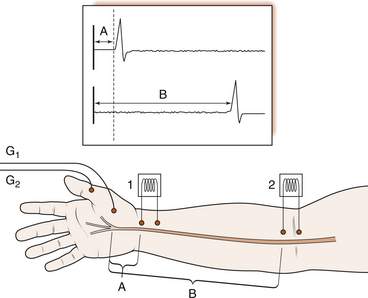
3 Michael Tonkin Peripheral nerves emerge from the spinal intervertebral foramina and travel to their endpoint structures, sensory receptors and neuromuscular junctions. The axon is a peripheral process from the nerve cell body in the anterior horn of the spinal cord (motor neuron) or the dorsal root ganglion (sensory neuron). Excitable cells such as neurons and muscle fibres communicate with each other at special regions called synapses. The first cell communicates with the second by releasing chemicals called neurotransmitters. The synapse between a motor neuron and a skeletal muscle fibre is called the neuromuscular junction, and the neurotransmitter is acetylcholine (see Ch. 8 for further discussion). Peripheral nerve fibres are usually classified into three types in relation to their conduction velocity, which is generally proportionate to size (Table 3.1). Nerve fibres are gathered into groups called fascicles and are surrounded by a mechanically strong membrane, the perineurium. Within the fascicles, nerve fibres lie within connective tissue called endoneurium. The fascicles themselves are embedded in an internal epineurium surrounded by an external loose epineurial connective tissue layer (Fig. 3.1). Nerves, such as the sciatic nerve, contain a greater percentage of connective tissue in relation to axonal substance. However, most people experience the sensation of leg and foot numbness following prolonged periods of sitting, especially on unyielding objects, when the protective benefit of the connective tissue cushion is overcome. The nerve trunk receives a segmental vascular supply. Extrinsic vessels run parallel to the nerve providing branches that lie within the epineurium, perineurium and endoneurium in a longitudinal pattern in each layer, with interconnecting branches between layers. Those vessels passing through the perineurium into the endoneurium often lie obliquely, creating a valve mechanism, which is vulnerable to pressure (Fig. 3.2). The endoneurial environment of the nerve is preserved by a combination of a blood–nerve barrier, in which the endoneurial vessels do not allow extravasation of proteins, and by the diffusion barrier of the perineurial sheath. The tissue pressure inside fascicles is slightly positive, providing a normal and healthy mechanical stiffness of fascicles. 1. Loss of the endoneurial layer (type III) 2. Loss of the perineurial layer (type IV) 3. Complete transection of the nerve with loss of the epineurial layers (type V). A nerve already damaged, either by a disease such as diabetic neuropathy or by a proximal compressive lesion, renders the nerve more irritable and more likely to be susceptible to compression at other sites. The latter lesion is sometimes called a double crush syndrome, as there are both proximal and distal lesions. Both nerve and muscle cells have a relative negative electrical charge inside them compared with the extracellular environment by virtue of a much higher concentration of potassium within the cells and a lower concentration of sodium and chloride. Electrical stimulation of the cells causes depolarization and generates an action potential. During depolarization, there is an opening of sodium channels in the cell membrane leading to an increase in sodium permeability and creation of an electric current by this rush of positively charged ions into the cell. Current then flows along the path of least electrical resistance, the length of the axon. Myelinated nerve fibres provide a mechanism for regenerating the charge of current (saltatory conduction). The myelin acts as an insulator to prevent current leakage. The myelin sheath indents at intervals, creating tight gaps that expose the axon, called nodes of Ranvier (Fig. 3.1). The action potential is propagated down the axon and exits at the node completing the electrical circuit through the extracellular fluid. This repeats the process of depolarization and perpetuates regeneration of the longitudinal current. However, there is a delay in the process at each node. Conduction velocity is faster with fewer nodal delays, the large-diameter nerves having the greatest internodal distances and therefore the fastest conduction speeds. These fibres include the alpha motor neurons and the sensory fibres transmitting light touch and proprioceptive (joint position) sensations. Pain and temperature and autonomic functions are conducted by slower, smaller myelinated or unmyelinated fibres. Motor nerve conduction studies assess the lower motor neurons from the level of the anterior horn to the muscle. The principle will be illustrated by reference to the median nerve (Fig. 3.3). A supramaximal electrical stimulus depolarizes all axons of the nerve and results in an action potential that travels in the normal physiological direction (orthodromically) down the nerve and is measured by recording electrodes overlying the thenar muscle belly. The distal motor latency is the time in milliseconds that it takes the impulse to travel from the stimulation point at the wrist to the recording electrode, say 3 milliseconds (ms). If the nerve is then stimulated at the elbow and the response follows after 7 ms, the motor conduction velocity is estimated by subtracting the distal motor latency from the proximal motor latency (i.e. 7−3=4 ms) and dividing the result by the distance between the two stimulating points (240 mm), i.e. a motor conduction velocity of 60 m/s. The shape of the wave is also important. A drop in amplitude indicates a conduction block, whereas an increase in duration indicates a lack of uniform conduction along the axons. Fig. 3.3 An electrical stimulus is given over the wrist (1) or elbow (2) and measured by recording electrodes at G overlying the abductor pollicis brevis muscle. The time taken to travel from 1 to G is the distal motor latency A; and from 2 to G is the proximal motor latency B. (based on Hilburn 1996, Fig. 4, p.212) Estimation of the F wave and H reflex provides additional information. The F wave is a late muscle response from the anterior horn cells in response to the same stimulus that evoked the early direct muscle response. It results in a discharge that sends an impulse back down the same motor axon. Thus the stimulus to the median nerve at the wrist resulted in a direct thenar muscle response after 3 ms and a later response, the F wave, giving the conduction time from the wrist to the spinal cord and back again. The F-wave latency gives an indication of the state of the nerve proximally and, if prolonged significantly, one may suspect proximal compression.
NERVE COMPRESSION SYNDROMES
Peripheral nerve anatomy
Pathophysiology and classification of nerve injury
Causes of nerve compression
Neuropathic
Electrophysiology
Nerve conduction studies
Motor nerve conduction studies
Sensory nerve conduction studies
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


NERVE COMPRESSION SYNDROMES
Only gold members can continue reading. Log In or Register to continue