This article reviews the indications for a muscle biopsy, and then gives a step-by-step description of the processes of muscle selection through to interpreting the biopsy report. The article aims to aid the clinician in preparing for a muscle biopsy procedure to avoid common pitfalls and obtain optimal results from this minimally invasive procedure. The basic anatomic structure of normal muscle is reviewed to provide a foundation for understanding common patterns of pathologic change observed in muscle disease and common and disease-specific histopathologic findings are presented, focusing on a select group of neuromuscular diseases.
- •
Muscle biopsy has an integral role in the diagnostic workup of patients presenting with quantifiable acute or chronic-progressive weakness.
- •
Electrodiagnostic testing, magnetic resonance or ultrasound imaging can aid in selection of an appropriate muscle for biopsy, and care should be taken to avoid biopsying muscle damaged by electromyography or trauma.
- •
There are 2 major categories of histopathologic abnormalities observed in muscle disease: neurogenic atrophy and myopathic changes. These structural changes often occur in tandem in myopathies.
- •
Immunohistochemical staining, biochemical testing, and electron microscopy can add to the diagnostic yield of routine muscle histology staining when indicated; however, the tissue samples must be handled and processed appropriately for each specialty technique to ensure optimal results.
- •
The best results are obtained from a muscle biopsy when there is good communication between the ordering clinician and the interpreting pathologist, the procedure is well thought out, and tissue retrieval, processing, and shipping are planned in advance to avoid common pitfalls.
Introduction
Muscle biopsy is an important tool for the evaluation and diagnosis of patients presenting to clinic with acute or progressive weakness who are suspected of having an underlying neuromuscular disorder. Alongside the clinical examination, electrodiagnostic, laboratory, and molecular genetic testing, muscle biopsy has a critical role, providing diagnostic evidence that either establishes the cause of disease or focuses the differential diagnosis. For example, in the setting of rapidly progressive muscle weakness, a muscle biopsy is the most expeditious diagnostic study to allow the clinician to distinguish between a necrotizing, metabolic, or inflammatory myopathy and facilitate rapid, appropriate therapeutic management. Or, as in the case of a young boy who presents with progressive proximal weakness and hyperckemia, and whose genetic tests do not confirm a dystrophinopathy, immunohistochemical staining of the muscle biopsy specimen can often identify the pathologic protein defect and pave the way for genetic confirmation of the disease.
The muscle biopsy itself is a straightforward procedure with little risk. However, to get the full benefit of the procedure several experts need to be involved, including a surgeon, processing laboratory and pathologist, which requires planning. Different from biopsies of other organs for which simple preservation in formalin is the routine procedure, a successful muscle biopsy requires optimal cryoprocessing of the fresh specimen to preserve viable macromolecules for enzyme histochemistry and metabolic assays. Therefore, the ordering physician must orchestrate the collection, packaging, and processing of tissues to ensure the desired testing can be performed, and to avoid the need for a repeat procedure because of limited, inappropriate, or poor sample quality. To this end, it is important that the ordering clinician is familiar with the procedure, knows the common pitfalls, and understands what each member of the team requires to provide an optimal outcome. Although it is not within the scope of this article to provide the depth of knowledge required of a neuropathologist to read and interpret a muscle biopsy, it is our hope that it provides the basic information needed to plan a biopsy procedure, instruct a team, get tissues successfully to the laboratory, and interpret the report once it is in hand.
Indications for muscle biopsy and muscle selection
We choose to do a muscle biopsy in the setting of quantifiable weakness and when we are certain that a diagnosis will not likely be reached in a less expensive, less invasive manner. For example, we order serum molecular genetic testing to rule out a dystrophinopathy before considering a muscle biopsy when presented with a male child who has progressive weakness, hyperckemia, calf hypertrophy, and who uses a Gower maneuver to stand. The same is true for the patient with classic signs and symptoms of myotonic muscular dystrophy type I, where the clinical examination is predictive of the diagnosis. However, in most cases, when the differential diagnosis is more extensive, we order a muscle biopsy in the early stage of care to home in on a diagnosis.
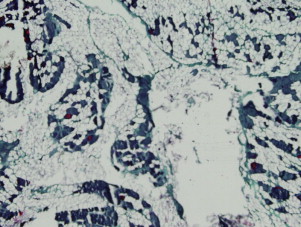
When choosing the site for biopsy, the most important step is to locate a muscle that is affected by the disease. Although this sounds simple, it is not always straightforward and can be challenging. If the disease process is chronic, progressive, and seems diffuse and symmetric, choosing the site for biopsy is typically easy and Medical Research Council (MRC) strength grading or electrodiagnostic testing can be used. Choosing a muscle with MRC grade 4/5 strength is often sufficient and provides tissue that reveals the disease and not just end-stage morphology. A muscle with MRC grade 3/5 strength is often too severely affected, with extensive nonspecific end-stage changes that may preclude identification of the muscle disease because of the lack of muscle fibers ( Fig. 1 ). However in acute-onset weakness, when there is little concern that end-stage disease is present, a muscle that is severely to moderately affected should be chosen.
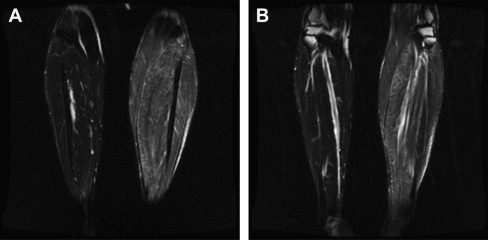
Electromyographic (EMG) testing can aid in identifying affected muscle; however, care should be taken to ensure that the biopsy is not performed on tissue with needle trauma from the examination, because this can also confound interpretation. Limiting the EMG study to a single side of the body allows the biopsy to be performed on a corresponding muscle on the opposite side. However, if the pathologic process seems patchy or multifocal, it is often better to use magnetic resonance imaging or ultrasonography to identify an involved muscle. When using EMG to locate affected muscle, if the disease is asymmetric, it is possible to inadvertently sample normal tissue. If you suspect an asymmetric process, imaging should be completed before the biopsy to confirm muscle involvement ( Fig. 2 ).
Muscles traditionally chosen for biopsy include the deltoid, biceps, and quadriceps. These muscles all have sufficient norms established for fiber type percentages and muscle fiber size for comparison. However, in a study by Lai and colleagues, the diagnostic usefulness of a biopsy taken from the deltoid proved superior to that of the biceps. The investigators surmised this was likely the case because of the proximal location of the deltoid muscle in the setting of myopathic diseases, which often have a proximal to distal gradient of muscle involvement. The gastrocnemius and tibialis anterior muscles are appropriate choices in diseases with distal limb signs and symptoms. The peroneus brevis muscle, located close to the superficial peroneal nerve, is a favored biopsy site when nerve biopsy is also indicated, as in the case of a suspected vasculitis.
The examining pathologist needs to be provided with information identifying the location of the biopsy site. Muscles vary in their normal ratio of type I to type II fibers, making this information necessary. In addition, the pathologist should have access to the patient’s medical history, including age, sex, physical condition, disease onset and progression, signs and symptoms, serum creatine kinase and other biochemical laboratory values, electrodiagnostic test results, medication and family history. Clear communication between the caring physician and the pathologist regarding clinicopathologic correlation is of vital importance for an accurate diagnosis or a list of differentials that points the way for the next step of care.
Indications for muscle biopsy and muscle selection
We choose to do a muscle biopsy in the setting of quantifiable weakness and when we are certain that a diagnosis will not likely be reached in a less expensive, less invasive manner. For example, we order serum molecular genetic testing to rule out a dystrophinopathy before considering a muscle biopsy when presented with a male child who has progressive weakness, hyperckemia, calf hypertrophy, and who uses a Gower maneuver to stand. The same is true for the patient with classic signs and symptoms of myotonic muscular dystrophy type I, where the clinical examination is predictive of the diagnosis. However, in most cases, when the differential diagnosis is more extensive, we order a muscle biopsy in the early stage of care to home in on a diagnosis.
When choosing the site for biopsy, the most important step is to locate a muscle that is affected by the disease. Although this sounds simple, it is not always straightforward and can be challenging. If the disease process is chronic, progressive, and seems diffuse and symmetric, choosing the site for biopsy is typically easy and Medical Research Council (MRC) strength grading or electrodiagnostic testing can be used. Choosing a muscle with MRC grade 4/5 strength is often sufficient and provides tissue that reveals the disease and not just end-stage morphology. A muscle with MRC grade 3/5 strength is often too severely affected, with extensive nonspecific end-stage changes that may preclude identification of the muscle disease because of the lack of muscle fibers ( Fig. 1 ). However in acute-onset weakness, when there is little concern that end-stage disease is present, a muscle that is severely to moderately affected should be chosen.
Electromyographic (EMG) testing can aid in identifying affected muscle; however, care should be taken to ensure that the biopsy is not performed on tissue with needle trauma from the examination, because this can also confound interpretation. Limiting the EMG study to a single side of the body allows the biopsy to be performed on a corresponding muscle on the opposite side. However, if the pathologic process seems patchy or multifocal, it is often better to use magnetic resonance imaging or ultrasonography to identify an involved muscle. When using EMG to locate affected muscle, if the disease is asymmetric, it is possible to inadvertently sample normal tissue. If you suspect an asymmetric process, imaging should be completed before the biopsy to confirm muscle involvement ( Fig. 2 ).
Muscles traditionally chosen for biopsy include the deltoid, biceps, and quadriceps. These muscles all have sufficient norms established for fiber type percentages and muscle fiber size for comparison. However, in a study by Lai and colleagues, the diagnostic usefulness of a biopsy taken from the deltoid proved superior to that of the biceps. The investigators surmised this was likely the case because of the proximal location of the deltoid muscle in the setting of myopathic diseases, which often have a proximal to distal gradient of muscle involvement. The gastrocnemius and tibialis anterior muscles are appropriate choices in diseases with distal limb signs and symptoms. The peroneus brevis muscle, located close to the superficial peroneal nerve, is a favored biopsy site when nerve biopsy is also indicated, as in the case of a suspected vasculitis.
The examining pathologist needs to be provided with information identifying the location of the biopsy site. Muscles vary in their normal ratio of type I to type II fibers, making this information necessary. In addition, the pathologist should have access to the patient’s medical history, including age, sex, physical condition, disease onset and progression, signs and symptoms, serum creatine kinase and other biochemical laboratory values, electrodiagnostic test results, medication and family history. Clear communication between the caring physician and the pathologist regarding clinicopathologic correlation is of vital importance for an accurate diagnosis or a list of differentials that points the way for the next step of care.
Open muscle biopsy procedure
A muscle biopsy is a small procedure that is best performed in a procedure room, but can be performed in a regular examination room if good lighting and a reasonable area for sterile equipment are ensured. Performing the procedure under general anesthesia is rarely indicated because of the risks and cost, but for young children and patients unable to remain still during the procedure, general anesthesia is often preferred. The essential equipment includes a self-retractor, scalpel, scissors, mosquito forceps, and pick-up forceps.
After identifying the biopsy site, the distal limb should be inspected. Pulses should be palpated and the limb assessed for signs of poor circulation. The skin overlying the muscle should be examined and the muscle palpated. If no concerning local or distal signs are seen in the limb, then the site should be prepared. First, the area should be cleaned with a surgical skin cleanser (eg, betadine or chlorhexidine). A sterile draping should then be placed around the surgical area. To achieve anesthesia in the skin and subcutaneous tissue, a local anesthetic should be injected, avoiding puncture and infiltration of the underlying muscle tissue ( Fig. 3 ). We routinely use 5 to 20 mL of 1% lidocaine. Lidocaine with epinephrine 1:100,000 effectively reduces bleeding. The epinephrine normally precludes the need for cautery or ligature. The anesthetic should be injected along the whole incision line to provide adequate anesthesia, which sets in within 2 minutes. The use of a buffered anesthetic removes much of the pain associated with this otherwise most painful part of the biopsy. The skin can then be incised with a scalpel ( Fig. 4 ). Skin is thicker in young adults compared with older adults, and proximal limbs have thicker covering than distal limbs. The subcutaneous adipose tissue, between the skin and muscle, is of varying thickness. When the subcutaneous tissue is deep, the length of the skin incision needs to be longer to allow reasonable exposure of the muscle. The subcutaneous tissue is best separated by blunt dissection and retracted ( Fig. 5 ). Beneath the subcutaneous tissue, the muscle is covered by a layer of fascia ( Fig. 6 ). Most human muscles do not have thick fascial layers, but the quadriceps muscle, a popular site for biopsy, has a thick fascia. If the muscle has a thick fascial covering, it needs to be incised using scissors or a blade to expose the muscle. When the muscle has been exposed, a group of fibers should be separated out bluntly from the belly of the muscle, avoiding the tendinous regions where muscle fibers normally are smaller, with increased connective tissue ( Fig. 7 ). It is helpful to surround the separated muscle fibers with a suture before cutting the ends of the fibers ( Fig. 8 ).
The amount of muscle tissue excised depends on planned testing and the nature of the underlying disease. If a multifocal or patchy disease process is suspected, multiple specimens may be needed to increase the likelihood of obtaining pathologic tissue. In general, a sample measuring 1 cm in length and 0.5 cm in diameter (about the size of 2 pencil erasers) excised in parallel to the length of the muscle fiber is adequate. Although many laboratories prefer working with clamped tissue, in which the sample is fixed in an isometric muscle clamp or stitched at both ends to a tongue blade or cork to keep it from retracting, we do not use a muscle clamp technique and have not had technical difficulties interfering with the quality of our biopsies. Check with your laboratory to determine if they prefer all or part of the specimen clamped.
If tissue needs to be sent for specialized testing, a larger sample may be required and the laboratory providing the processing should be contacted for specifications ( Table 1 ). Once removed, the specimen should be inspected ( Fig. 9 ) and then packaged as described in the section on shipping and handling.
| Assessment Technique | Use | Ideal Amount of Tissue | Immediate Processing Requirements |
|---|---|---|---|
| Frozen section | Muscle fiber morphology and enzyme histochemistry Most diagnostic information with light microscopy | 1 cm 3 | Wrap in lightly moist gauze and ship to processing laboratory |
| Paraffin embedding | Inflammation and morphology of inflammatory cells | 0.5 cm x 1 cm | Wrap in lightly moist gauze and ship to processing laboratory |
| EM | Ultrastructural analysis Visualization of endomysial capillaries, inclusions, mitochondria, myofilaments, collagen, and so forth | 1–2-mm-thick section | 4% glutaraldehyde |
| Biochemical testing | Assessing storage and mitochondrial diseases | 50–550 mg of tissue, but depends on anticipated testing | Rapid freezing in liquid nitrogen at site of biopsy. Ship frozen on dry ice overnight |
Bleeding at the surgical site should be stopped by irrigation with epinephrine and applied pressure. If bleeding continues, then cautery or ligation should be performed. Before beginning closure, it is necessary that hemostasis is achieved. If fascia was sectioned, it should be sutured ( Fig. 10 ) to prevent muscle herniation, which otherwise can be a chronic nuisance for the patient. After closing the fascia, the subcutaneous tissue should be sutured and the skin closed. We use 4:0 or 3:0 resorbable suture material to close the fascia, subcutaneous tissue, and the skin ( Figs. 11 and 12 ). After skin closure, the biopsy site should be bandaged. We normally use Steri-Strips (3M USA, St. Paul, MN, USA), nonstick gauze, and occlusive dressing ( Fig. 13 ). If the site can be wrapped with an elastic wrap, a light pressure bandage can be applied and left on for a few hours.








