CHAPTER 20 Molecular and cellular regulators
1. Describe the importance of adhesion and the migration of leukocytes in inflammation.
2. Distinguish between growth factors and cytokines.
3. Identify important processes in wound healing that are regulated by growth factors, cytokines, proteases, or hormones.
4. Describe the molecular environment that growth factors need to promote wound healing.
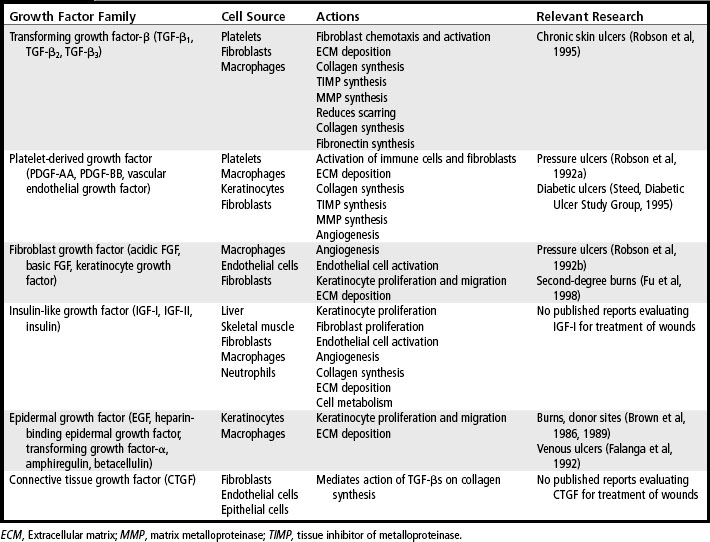
5. For each growth factor family, list one member, a key target cell, one main action, and one therapeutic use.
6. Describe the molecular differences among acute healing wounds and chronic nonhealing wounds.
7. Correlate the application of the principles of wound bed preparation with the removal of wound healing barriers.
Biologic roles of cytokines and growth factors
General phases of wound healing
The phases of wound healing are hemostasis, inflammation, proliferation and repair, and remodeling. There is considerable temporal overlap of these phases of healing, and the entire process lasts for several months. Immediately after injury the process of blood clotting is initiated by activation of a proteolytic cascade, which ultimately converts fibrinogen into fibrin. As the fibrin molecules self-associate into a weblike net, red blood cells (RBCs) and platelets become entrapped. The aggregate of fibrin, RBCs, and platelets quickly grows large enough to form a tampon that blocks an injured capillary and stops the flow of blood.
Adhesion molecules and adhesion receptors in inflammation
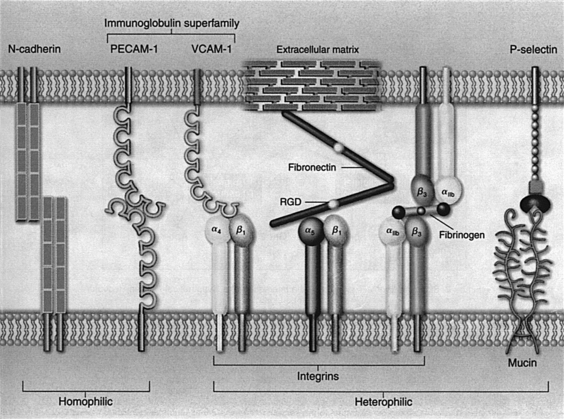
The chemotactic attraction of leukocytes to a wound and the movement of leukocytes from the blood into wounded tissue (extravasation) involve expression and activation of adhesion molecules and adhesion receptors on leukocytes, platelets, and vascular endothelial cells. Cytokines and growth factors play key roles in these processes (Arai et al, 1990; Frenette and Wagner, 1996a, 1996b; Springer, 1990). Among the many types of adhesion molecules and receptors on the cell surface, four major families of transmembrane proteins stand out in the process of inflammation: integrins, selectins, cell adhesion molecules, and cadherins (Figure 20-1).
Integrins are glycoproteins composed of two different types of subunits, designated α and β. In simple terms, integrins are cellular receptors for extracellular matrix proteins, as shown with α5β1, which is a receptor for fibronectin. A short amino acid sequence, such as arginine-glycine-aspartate (RGD), is often the site of recognition by the integrin receptor. Integrins are important because they are capable of generating signals inside cells when the integrin receptor binds to a specific extracellular matrix protein, in much the same way the insulin receptor generates intracellular signals, which regulate glucose transport into a cell when insulin binds to its cellular receptor. Expression of β2 integrins is limited to leukocytes, whereas β1 integrins are expressed on most cell types. β1 integrins primarily bind to extracellular matrix components such as fibronectin, laminin, and collagens. (These substances are discussed in more detail in Chapter 4.)
Selectins are proteins that have a unique structure called a lectin domain at the distal end, which can bind specific carbohydrate groups of glycoproteins or mucins on adjacent cells. Thus, unlike other adhesion proteins, which recognize specific protein structures, selectins recognize and bind to carbohydrate ligands on leukocytes and vascular endothelial cells. E-selectin appears on endothelial cells after they have been activated by inflammatory cytokines, and P-selectin is stored in the α-granules of platelets and the storage granules of endothelial cells (Weibel-Palade bodies).
During the process of extravasation of inflammatory cells into a wound, important interactions occur between blood vessels and blood cells (Arai et al, 1990; Frenette and Wagner, 1996a, 1996b; Springer, 1990). Initially, circulating leukocytes begin rolling on endothelial cells through the binding of glycoproteins expressed on their cell surface to selectins, transiently expressed by activated endothelial cells of venules (Figure 20-2). The binding affinity of selectins is relatively low but is enough to serve as a biologic brake, making leukocytes quickly decelerate by rolling on endothelial cells. While rolling, leukocytes can become activated by chemoattractants (cytokines, growth factors, or bacterial products). After activation, leukocytes firmly adhere to endothelial cells as a result of the binding between their β2 class of integrins and ligands, such as VCAM and ICAM expressed on activated endothelial cells. Chemotactic signals present outside the venule induce leukocytes to squeeze between endothelial cells of the venule and migrate into the inflammatory center by using their β1 class of integrins to recognize and bind to extracellular matrix components.
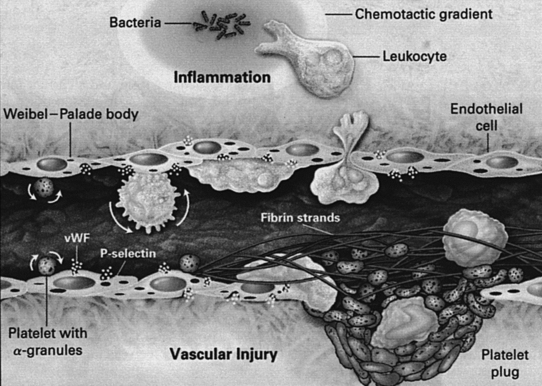
FIGURE 20-2 Interactions between blood cells and a stimulated or injured venule.
(From Frenette PS, Wagner DD: Adhesion molecules. Part II: Blood vessels and blood cells, N Engl J Med 335:43, 1996.)
Inflammatory cell proteases
When the inflammatory cascade is activated, neutrophils enter the wound initially, followed by macrophages. Neutrophils and macrophages become activated and engulf and destroy bacteria through their production of reactive oxygen species (super oxide anion, oxygen free radicals, or hydrogen peroxide). Activated neutrophils and macrophages also release several proteases, including neutrophil elastase (a serine type of protease), neutrophil collagenase (a matrix metalloproteinase type of protease designated MMP-8), and macrophage metalloelastase (MMP-12). These proteases play important, beneficial roles in initiating normal wound healing by removing (proteolytically degrading) damaged extracellular matrix components, which must be replaced by new, intact extracellular matrix molecules for wound healing to proceed. These proteases also are important for enabling inflammatory cells to move through the basement membrane that surrounds capillaries.
Inflammatory cell cytokines and growth factors in proliferation and repair
The growth factors released by platelets diffuse away from a wound within a few hours, but they are replaced by growth factors and cytokines that are produced by neutrophils, macrophages, activated fibroblasts, vascular endothelial cells, and epidermal cells that are drawn into the wound area. For example, activated macrophages secrete several important cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), which have a variety of actions on different cells. TNF-α and IL-1β are potent inflammatory cytokines, which further stimulate inflammation. TNF-α also induces macrophages to produce IL-1β, which is mitogenic for fibroblasts and up-regulates expression of MMPs. Both TNF-α and IL-1β directly influence deposition of collagen in the wound by inducing synthesis of collagen by fibroblasts and by up-regulating expression of MMPs. In addition, these cytokines down-regulate expression of the tissue inhibitors of metalloproteinases (TIMPs), which are the natural inhibitors of MMPs. Interferon-γ (IFN-γ), produced by lymphocytes attracted into the wound, inhibits fibroblast migration and down-regulates collagen synthesis (Table 20-1).
Inflammatory cells secrete other growth factors, including TGF-β, TGF-α, heparin-binding epidermal growth factor (HB-EGF), and basic fibroblast growth factor (bFGF). The growth factors secreted by macrophages continue to stimulate migration of fibroblasts, epithelial cells, and vascular endothelial cells into the wound. As the fibroblasts, epithelial cells, and vascular endothelial cells migrate into the site of injury, they begin to proliferate, and the cellularity of the wound increases. This begins the proliferative and repair phase, which often lasts several weeks. If the wound is not infected, the number of inflammatory cells in a wound begins to decrease after a few days. Other types of cells, such as fibroblasts, endothelial cells, and keratinocytes, are drawn into the wound and begin to synthesize growth factors. Fibroblasts secrete IGF-I, bFGF, TGF-β, PDGF, and keratinocyte growth factor (KGF). Endothelial cells produce vascular endothelial cell growth factor (VEGF), bFGF, and PDGF. Keratinocytes synthesize TGF-α, TGF-β, and IL-1β. These growth factors continue to stimulate cell proliferation and synthesis of extracellular matrix proteins and to promote formation of new capillaries.
Cytokines
Cytokines are produced extensively by activated T cells and macrophages, although nonimmune system cells such as keratinocytes and vascular endothelial cells also produce some cytokines. Studies have revealed that cytokines generally induce multiple biologic activities (pleiotropic) and that a single cytokine can act as both a positive signal and a negative signal, depending on the type of the target cell. Cytokines such as IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, and IL-10, granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), IFN-γ, and TNF-α are key mediators of immune and inflammatory responses. A cytokine is also referred to as a lymphokine (cytokine made by lymphocytes), monokine (cytokine made by monocytes), chemokine (cytokine with chemotactic action), and interleukin (cytokines made by one leukocyte and acting on other leukocytes). Two cytokines in particular, TNF-α and IL-1β, have activities that substantially influence skin wound healing through their ability to increase production of MMPs and suppress production of TIMPs. Table 20-2 lists the cytokines involved in wound healing along with cell source, biologic activity, and their subclassification as proinflammatory or antiinflammatory.
TABLE 20-2 Cytokines Involved in Wound Healing
| Cytokine | Cell Source | Biologic Activity |
|---|---|---|
| Proinflammatory Cytokines | ||
| TNF-α | Macrophages | PMN margination and cytotoxicity; collagen synthesis; provides metabolic substrate |
| IL-1 | Macrophages Keratinocytes | Fibroblast and keratinocyte chemotaxis; collagen synthesis |
| IL-2 | T lymphocytes | Increases fibroblast infiltration and metabolism |
| IL-6 | Macrophages PMNs Fibroblasts | Fibroblast proliferation; hepatic acute-phase protein synthesis |
| IL-8 | Macrophages Fibroblasts | Macrophage and PMN chemotaxis; keratinocyte maturation |
| IFN-γ | T lymphocytes Macrophages | Macrophage and PMN activation; retards collagen synthesis and cross-linking; stimulates collagenase activity |
| Antiinflammatory Cytokines | ||
| IL-4 | T lymphocytes Basophils Mast cells | Inhibition of TNF, IL-1, IL-6 production; fibroblast proliferation; collagen synthesis |
| IL-10 | T lymphocytes Macrophages Keratinocytes | Inhibition of TNF, IL-1, IL-6 production; inhibits macrophage and PMN activation |
IFN, Interferon; IL, interleukin; PMN, polymorphonuclear leukocyte; TNF, tumor necrosis factor.