CHAPTER 4 Modulation of visceral function by somatic stimulation
Introduction
At the most superficial level, we may ask how somatic stimuli produce effects on visceral function. In fact, various practices, such as osteopathy and chiropractic, have gravitated towards an attractive explanation for this phenomenon: reflex neurological mechanisms, especially those involving the autonomic nervous system. This collective, intuitive evolution in thinking is addressed by a number of authors elsewhere in this book. We may also ask how it is that, as observed by practitioners of various disciplines, stimulation at a particular somatic site reliably produces physiological responses at a specific distant visceral site. This topographical mapping of stimulation points onto target viscera is most systematized in acupuncture, but is also seen in chiropractic and osteopathy, wherein some practitioners have related chiropractic subluxation or somatic lesion at one level of the spine to symptoms in specific organs as discussed in Chapter 12.
Thus, this chapter will first define and examine somatovisceral reflexes, drawing primarily on work involving anesthetized animals. We will proceed to look in more detail at somatovisceral reflexes evoked by stimulation of musculoskeletal structures, and then consider a specific subclass of somatovisceral reflexes, the spinovisceral reflexes. For convenience, we have focused on cardiovascular effects. However, the underlying principles can be applied equally to other organ systems (Fig. 4.1; see also Chapter 1).
Somatovisceral reflexes
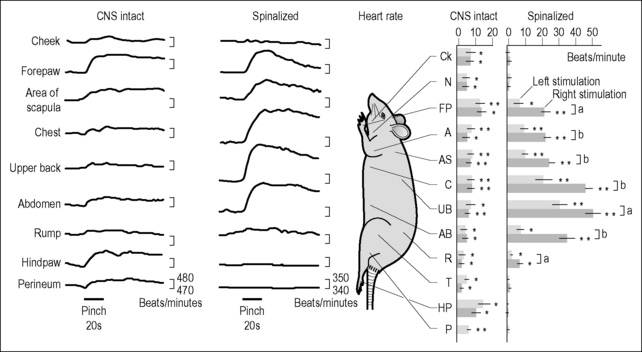
An excellent paper which demonstrates many of the cardinal features of somatovisceral reflexes (Kimura et al. 1995) examined blood pressure and heart rate responses to noxious mechanical cutaneous stimulation (pinching) applied to different dermatomes in the anesthetized rat (Fig. 4.1). Noxious stimulation resulted in changes in sympathetic motor neuron activity which corresponded to the magnitudes of the effects on blood pressure and heart rate. More specifically, pinching induced increases in heart rate which were matched by increases in cardiac sympathetic nerve activity. Similarly, increases in blood pressure were accompanied by corresponding increases in renal sympathetic nerve activity. In central nervous system-intact (CNS-intact) animals the segmental organization of these reflexes was not well resolved. However, in spinalized animals – animals whose cervical spinal cords had been cut – it was immediately apparent that much more profound effects were achieved when stimulation was applied at or immediately adjacent to the segmental levels of sympathetic outflow to the target end-organs. In fact, with heart rate there was even a clear laterality to the response, with stimulation to the right side of the animal producing significantly larger effects than stimulation to the left. Thus, with noxious cutaneous stimulation, at least in the spinalized animal, the somatotopic relationships between site of stimulation and end-organ response are well established and well accounted for by somato-autonomic reflex mechanisms.
Somatovisceral reflexes elicited by stimulation of musculoskeletal tissues
Of equal or greater relevance to the somatic therapies are studies involving stimulation – particularly innocuous mechanical stimulation – of musculoskeletal structures. Earlier studies examined responses to limb muscle stimulation where results are likely to be less precisely defined. Nonetheless, we have studies demonstrating cardiovascular responses to innocuous stimulation (stretch and compression) of limb muscles in anesthetized animals (Tallarida et al. 1981). Several studies have demonstrated the effects of massage and pressure-point therapy on autonomic tone and cardiovascular function in healthy volunteers and patients (Delaney et al. 2002, Mok & Woo 2004, Wang & Keck 2004). Similar effects have also been achieved with acupuncture (Lin et al. 2003, Nishijo et al. 1997), although results vary quantitatively and qualitatively according to the acupuncture point used and the parameters of stimulation.
Effects on heart rate and blood pressure are easily monitored and of considerable clinical interest. However, we also have demonstrations in animal models of innocuous somatosensory stimulation influencing muscle, nerve, skin, gastric and hepatic blood flow, as well as gastric, duodenal and jejunal motility, urinary bladder motility, adrenal and pancreatic secretion, and immune functions of various sorts (see Sato et al. 1997).
Thus we observe that under laboratory and clinical conditions somatic stimulation, including innocuous stimulation, induces changes in the activity of autonomic motor neurons and hence changes in the physiology of the dependent end-organs. Occasionally in CNS-intact animals, and often in spinalized animals, there is a clear somatotopic relationship between the site of stimulation and the end-organ that is principally affected. In spinalized animals, stimulation is likely to produce its most vigorous responses in end-organs whose autonomic innervation originates at or very close to the spinal segment receiving the sensory input. As increasing numbers of patients are surviving spinal cord injuries, parallel investigations may soon be practical in humans. The phenomenon of sympathetic dysreflexia seen in patients with high spinal cord injuries provides an unfortunate demonstration that spinally mediated somatosympathetic reflexes do indeed exist in humans. It remains to be seen whether a segmental organization to these reflexes can be demonstrated in patients with chronic spinal cord injuries (but see Burton et al. 2008).
Spinovisceral reflexes
Spinovisceral reflexes are a subclass of somatovisceral reflex wherein stimulation delivered to paraspinal or spinal tissues evokes changes in visceral function. A familiar example of a spinovisceral reflex would be paralytic ileus – the loss of bowel motility that accompanies significant injury to the lower spinal column. Spinovisceral reflexes have particular significance in the disciplines of osteopathy and chiropractic. Historically – albeit less so today – practitioners of these disciplines have maintained that a biomechanical lesion at a particular level of the spinal column is most likely to produce symptoms in a specific visceral organ, especially an organ that receives autonomic innervation from the same level of the spine as the lesion (Burns et al. 1948).
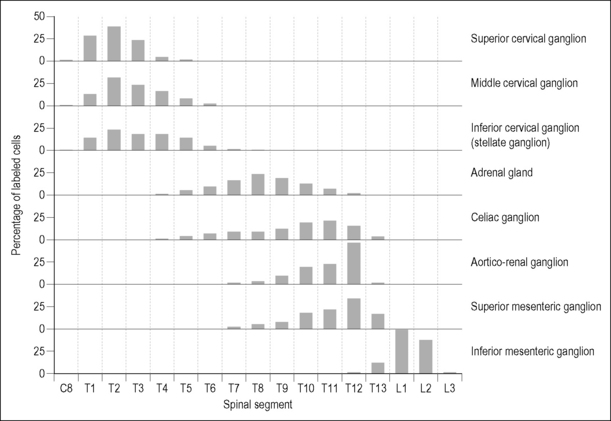
The appeal of putative somato-autonomic reflex mechanisms is largely due to the clear segmental organization of the peripheral sympathetic nerves: something that is obvious even to those with no more than a textbook acquaintance with human anatomy. This anatomical feature is seen as providing a scientific rationale for the clinical observation of reliable topographical relationships between site of somatic dysfunction and visceral disorder (see Chapter 1, Fig. 4.1 and Chapter 12). The internal structure of the spinal cord, where the autonomic motor neurons originate, also has a clear segmental organization. The motor neurons of the spinal sympathetic nuclei are not distributed in continuous columns (Cabot 1990), but rather are arranged as linked nests in the thoracic and upper lumbar spinal cord (Fig. 4.2). Axons from each nest of sympathetic preganglionic neurons (SPNs) exit the spinal cord mainly through the nerve roots originating at their level and project to discrete peripheral targets (Oldfield & McLachlan 1981). Hence, in the rat, more than 95% of SPNs projecting to the middle cervical ganglion originate between the T1 and T5 spinal segments, whereas more than 95% of SPNs to the adrenal medulla originate between T5 and T11 (Strack et al. 1988). Studies in other species and using different labeling methods have shown even more restricted viscerotopic clustering of SPNs (Rubin & Purves 1980). Upper thoracic SPNs project to the heart, particularly the ventricles, via the middle cervical ganglion and stellate ganglion. In the cat, preganglionic neurons to the cervical sympathetic trunk originate almost exclusively in the upper five thoracic segments of the spinal cord (Oldfield & McLachlan 1981).
The cell bodies of postganglionic neurons within the stellate ganglion are also arranged in a somatotopic fashion so that, in the cat, neurons projecting to the heart are clustered in the middle part of the ganglion (Masliukov et al. 2000). These cells are morphologically distinct from stellate ganglion cells projecting to other organs such as the sternocleidomastoid muscle. The size and number of stellate ganglion cells to the heart increases shortly after birth, but by 1 month the number of neurons to the heart begins to decrease as cells that have failed to make functional connections undergo apoptosis (Masliukov et al. 2000).
In humans it is also apparent that spinal preganglionic neurons and paravertebral ganglia have specific projections, and changes in output of particular peripheral autonomic nerves are associated with specific changes in peripheral organ function (Armour 1972, Hageman 1973, Haws & Burgess 1978, Schwartz et al. 1976). Hence, the gross anatomy and fine structure of the autonomic nervous system, and physiological investigations, support the concept of a segmental and even lateral organization to somatovisceral reflexes, stimuli at particular sites preferentially affecting specific organs.
So far, we have concentrated on the efferent arm of the somato-autonomic reflex. However, the segmental anatomy of the sympathetic nervous system is mirrored to a degree by the obviously segmental anatomy of the spinal column and its afferent innervation. It has been demonstrated that the functional sensory innervation of the spinal column from each spinal nerve is limited to no more than three vertebral levels in the rat (Budgell et al. 1997a). In humans, anatomical evidence suggests a similar or perhaps somewhat more limited divergence of spinal afferents (Bogduk & Twomey 1991). Therefore, of all of the tissues in the human body, it is the spinal structures that appear most likely to elicit a segmentally organized – i.e. organ-specific – reflex response.
Animal model studies that have explored nociception associated with afferents innervating the spine demonstrate that noxious stimulation of spinal tissues causes changes in visceral function, and that these responses often show a segmental organization. Among the clinically relevant animal models of spinovisceral reflexes is interspinous microinjection of algesics. One such algesic, capsaicin, binds to a specific subclass of polymodal nociceptors (primarily TRPV1-receptor) and produces an intense noxious stimulation which may last for 20–30 minutes. In anesthetized rats, capsaicin has been injected into the interspinous tissues (also called the interspinous ligament) at specific spinal levels, and effects have been observed in sympathetic nerve activity and visceral function. This model has been used to demonstrate spinovisceral reflex effects on cardiac function and sciatic nerve blood flow (Budgell et al. 1995), adrenal nerve activity and catecholamine secretion (Budgell et al. 1997b), urinary bladder motility (Budgell et al. 1998), and gastric motility (Budgell & Suzuki 2000). Especially in spinalized animals, the reflex response is apparently facilitated when stimulation is applied to afferents entering the spinal cord at or close to the level of sympathetic efferent output to the target organ. Responses to vehicle injection alone were insignificant, confirming that the reflex responses were particularly elicited by the noxious stimulation of the algesic. As with Kimura’s work, cited above (Kimura et al. 1995), responses were shown to be centrally mediated, at both the spinal and the supraspinal levels, and related predominantly to changes in sympathetic motor neuron activity.
Controlled innocuous mechanical stimulation of paraspinal muscles is technically challenging. However, as outlined in Chapter 3, Pickar and colleagues have produced a series of interesting studies involving lumbar paraspinal stimulation in the anesthetized cat. In brief, Pickar and colleagues have investigated the interaction of mechanical loading of the lumbar spine and noxious chemical stimulation of paraspinal muscles as they effect splenic and renal sympathetic nerve activity, heart rate, and blood pressure (Kang et al. 2003). In α-chloralose-anesthetized CNS-intact cats, they determined that noxious chemical stimulation of the L2–4 multifidus muscles, using mustard oil, led to complex responses (elevation followed by depression) in blood pressure, with concomitant and sustained increases in heart rate, and splenic and renal sympathetic nerve activity. These responses were mediated at the supraspinal level and were modified by loading the L3 spinous process with a force equivalent to 100% of the animal’s body weight directed from posterior to anterior. The magnitudes of splenic and renal sympathetic nerve reflex responses were somewhat attenuated, but remained elevated longer in the presence of mechanical loading. However, mechanically loading the spine in the absence of noxious stimulation produced no significant changes in the parameters measured. These results suggest that, within this experimental system, relatively forceful but innocuous mechanical loading of the spine is ineffective in eliciting somato-autonomic reflexes. These results are consistent with the majority of laboratory observations to date, noting generally clear and reproducible reflex autonomic responses to noxious stimulation, but weak and inconsistent responses to innocuous mechanical stimulation.