Modular Liner Exchanges for the Treatment of Osteolysis
Anders Troelsen
Craig J. Della Valle
Nanna H. Sillesen
Matthew J. Dietz
Young-Min Kwon
Harry E. Rubash
Henrik Malchau
Case
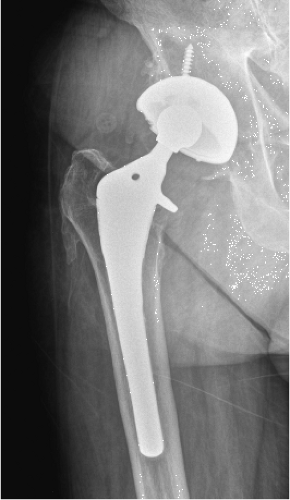
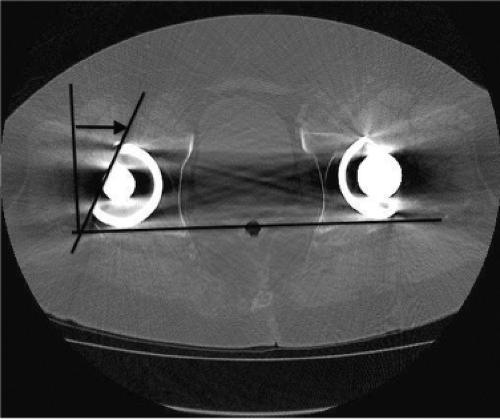
A 73-year-old male presented 18 years following a primary total hip arthroplasty (THA) performed for osteoarthritis; the bearing surface included standard (not highly cross-linked) polyethylene. The patient is very active walking more than 2 miles per day and reports engaging in doubles tennis regularly as well as skiing. He had done well until the last year, when he began noticing pain in the groin. He has not seen the surgeon who performed his THA in more than 10 years as he was doing so well. Plain x-rays showed well-fixed cementless acetabular components in place with evidence of severe retroacetabular and mild proximal femoral osteolysis (Fig. 117.1). Evaluation for infection included a normal ESR and CRP. A CT scan performed confirmed the extensive osteolysis and good positioning of the femoral and acetabular components with appropriate anteversion (Fig. 117.2).
Introduction
Osteolysis and loosening of femoral and acetabular components is a major cause of late revisions in THA. Wear of the acetabular liner with particle formation and the biologic
response to this debris has been recognized as the process leading to osteolysis. During revision of the acetabular component with preoperative signs of radiographic osteolysis, there are two potential strategies for treatment: (1) revision of the cup with complete removal and grafting of bony defects or (2) modular liner exchange. Both strategies have unique theoretical advantages and disadvantages influencing the clinical outcome, the rate of complications, and survivorship of the THA. This chapter will focus on modular liner exchange for treatment of osteolysis.
response to this debris has been recognized as the process leading to osteolysis. During revision of the acetabular component with preoperative signs of radiographic osteolysis, there are two potential strategies for treatment: (1) revision of the cup with complete removal and grafting of bony defects or (2) modular liner exchange. Both strategies have unique theoretical advantages and disadvantages influencing the clinical outcome, the rate of complications, and survivorship of the THA. This chapter will focus on modular liner exchange for treatment of osteolysis.
Historically and currently, polyethylene modular liners are the most frequently used bearing surfaces in THA. Although polyethylene wear debris is associated with osteolytic development, it is important to acknowledge that polyethylene bearing surfaces have undergone important developments, cross-linking and vitamin E infusion, resulting in high wear resistance of the polyethylene which may decrease the risk of this complication in the future.
Polyethylene particles generated from total joint replacement are created from various types of wear. Wear is the consequence of an articulation between two surfaces which result in removal of material. The way in which the hip is inserted combined with how the patient uses their artificial hip, will both determine the mechanical motion with which the polyethylene liner wears in vivo.
The artificial hip intended articulation is between the prosthetic head and the liner in a concentric pattern inside the polyethylene dome (Fig. 117.3). However, the articulation can also be between an intended articulating surface and an unintended surface, as for example edge loading/eccentric wear between the head and the rim of the liner (Fig. 117.4), the head against the metal backshell, (Fig. 117.3) or the neck of the head against the rim as seen in impingement (Fig. 117.5). Accelerated wear may occur with regularly loading the hip with more extreme activities (such as running), or if third-body debris are interpositioned between the two primary articulating surfaces which can cause fretting and scratching of the polyethylene liner and the metal head (Fig. 117.6). Orishimo et al. reported a fourfold increase in the likelihood of osteolysis for every 0.1-mm increase in the annual linear wear rate. This group also found that a wear rate of 0.2 mm per year as a critical threshold for the development of osteolysis (1).
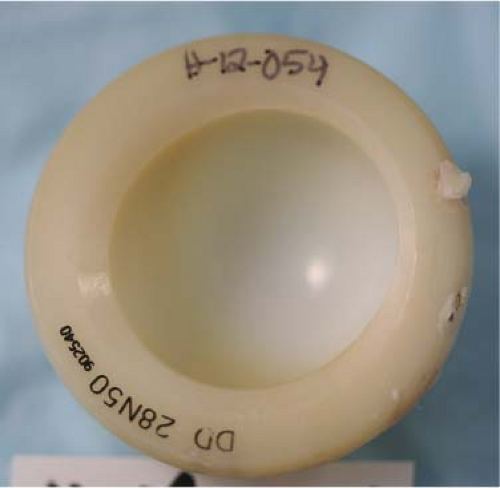 Figure 117.3. Concentric loading. Notice the imprint in the dome and the slight spherical opening due to >10 years of wear. Conventional polyethylene. |
Charnley described the periprosthetic osteolysis in 1975. He found the presence of cystic lesions in the bone around a femoral fracture prosthesis, and that these findings were associated with a high volume of macrophages (2). Research has later confirmed particulate debris in various tissue samples from revision surgeries and autopsies, and that wear particles are involved in the inflammatory process (3,4). The cellular mechanism of osteolysis is, in part, due to activation of monocytes by the particles in the periprosthetic tissue.
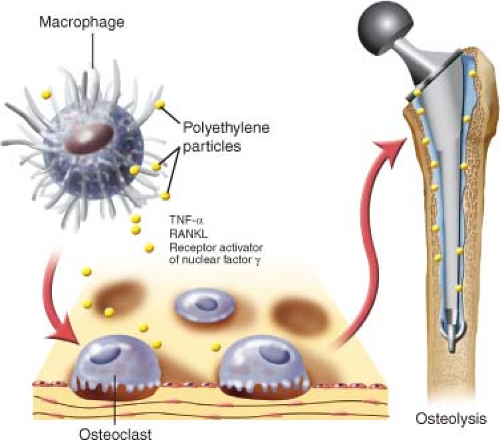
The activated macrophages release a cascade of cytokines that again activate other inflammatory cells, and thereby further release of cytokine and chemokines which recruit osteoclasts to the site and stimulate bone resorption (5,6,7). The osteolytic areas have a high frequency of osteoclasts and osteoblasts both known for their mutual involvement in bone remodeling and bone turnover, but the presence of cytokines suppresses the osteoblasts’ functions leading to more osteolysis than bone formation (8,9,10,11). Once an osteolytic lesion is formed in response to inflammation, it is replaced by remodeled fibrous tissue and lymphocytes, macrophages, and fibrohistiocytes. The fibroblasts are facultative phagocytes and their activation can accelerate the osteolytic process by the release of enzymes that degrade the organic components of the bone (12,13,14,15). Immunohistochemical analysis of tissue obtained at revision surgery found that tumor necrosis factor-alpha (TNF-α), receptor activator of nuclear factor-κ ligand (RANKL), and receptor activator of nuclear factor-κ (RANK) were all highly expressed by large multinucleated cells containing polyethylene particles (Fig. 117.7) (14). TNF-α can enhance the RANKL activity, and the RANKL/RANK-ligand–receptor complex stimulates the osteoclast differentiation and activity (8).
The activated macrophages release a cascade of cytokines that again activate other inflammatory cells, and thereby further release of cytokine and chemokines which recruit osteoclasts to the site and stimulate bone resorption (5,6,7). The osteolytic areas have a high frequency of osteoclasts and osteoblasts both known for their mutual involvement in bone remodeling and bone turnover, but the presence of cytokines suppresses the osteoblasts’ functions leading to more osteolysis than bone formation (8,9,10,11). Once an osteolytic lesion is formed in response to inflammation, it is replaced by remodeled fibrous tissue and lymphocytes, macrophages, and fibrohistiocytes. The fibroblasts are facultative phagocytes and their activation can accelerate the osteolytic process by the release of enzymes that degrade the organic components of the bone (12,13,14,15). Immunohistochemical analysis of tissue obtained at revision surgery found that tumor necrosis factor-alpha (TNF-α), receptor activator of nuclear factor-κ ligand (RANKL), and receptor activator of nuclear factor-κ (RANK) were all highly expressed by large multinucleated cells containing polyethylene particles (Fig. 117.7) (14). TNF-α can enhance the RANKL activity, and the RANKL/RANK-ligand–receptor complex stimulates the osteoclast differentiation and activity (8).
The radiolucent regions of osteolysis that develop around the acetabular metal backshell is apparent in both cemented and uncemented sockets (16,17,18). However, the osteolytic appearance differs depending on whether the component was placed with or without cement. In the cemented sockets, the radiograph will typically show a linear pattern, with a clear space between the bone–cement interface primarily in DeLee–Charnley zone III or I (16,19). The uncemented socket shows a different pattern of radiolucency, primarily in DeLee–Charnley zone II and III, and with an onset from the metal socket–bone interface, appearing as cystic formations into the cancellous bone (16). However, the acetabular socket might still be well fixed to the bone due to bone ingrowth onto the metal shell in other nonosteolytic areas of the dome (20,21). A postmortem study found that debris granulomas extended into the bone-like balloons from the socket screw holes and were present in the histologic postmortem samples after 6 years of implantation. These lesions had not been visible on x-rays and all of the sockets had been stable (22).
Radiographic Diagnosis of Osteolysis
Patients seen for routine follow-up or evaluated for hip pain following THA have routine conventional radiographs taken as the first line of radiographic assessment. Usually an anteroposterior pelvic and a lateral view are recorded. Osteolytic lesions appear as radiolucent (dark on radiographs) rounded areas based on the implant and expanding into the periacetabular bone. Repeated radiographs can be used to monitor the development of these osteolytic lesions. Care should be taken that repeated radiographs are taken using the same radiographic standard as differences in views will obstruct comparison of radiographs. Further, conventional radiography underestimates the size of osteolytic lesions when compared to findings on CT scans or intraoperative findings (23,24,25,26). CT scanning also allows the surgeon to precisely determine the anteversion of the cup and stem, which is critical given the high incidence of dislocation following revision procedures in general and isolated liner exchanges in particular. Identification of components that are inadequately or overly anteverted can assist with preoperative planning and the decision to retain or revise the cup or stem.
Indications and Contraindications
Faced with a diagnosis of acetabular osteolysis, assessment of the cup stability is crucial for the choice of treatment
strategy. If a cup is loose, it must be revised. Surgical decision making is often difficult in cases of uncemented cups presenting with osteolysis and no clinical symptoms. The lack of symptoms may indicate that the cup is still stable. However, given the expansile pattern of osteolysis in uncemented cups, it likely will progress until the cup becomes loose, resulting in persisting hip pain, and potentially severe bone loss. Treatment strategies dealing with acetabular osteolysis in the setting of a stable cup include either retention or revision of the cup. During this decision making the following has to be considered: (1) How is the cup positioned?; (2) has the cup shown acceptable implant survivorship?; (3) is it a modular component?; (4) is the cup damaged?; (5) is the liner locking mechanism intact?; (6) can a new polyethylene liner of sufficient thickness be inserted? (27,28). Based on these criteria, Rubash et al. suggested a classification scheme and treatment algorithm dealing with pelvic osteolysis in THA (Fig. 117.8) (27



strategy. If a cup is loose, it must be revised. Surgical decision making is often difficult in cases of uncemented cups presenting with osteolysis and no clinical symptoms. The lack of symptoms may indicate that the cup is still stable. However, given the expansile pattern of osteolysis in uncemented cups, it likely will progress until the cup becomes loose, resulting in persisting hip pain, and potentially severe bone loss. Treatment strategies dealing with acetabular osteolysis in the setting of a stable cup include either retention or revision of the cup. During this decision making the following has to be considered: (1) How is the cup positioned?; (2) has the cup shown acceptable implant survivorship?; (3) is it a modular component?; (4) is the cup damaged?; (5) is the liner locking mechanism intact?; (6) can a new polyethylene liner of sufficient thickness be inserted? (27,28). Based on these criteria, Rubash et al. suggested a classification scheme and treatment algorithm dealing with pelvic osteolysis in THA (Fig. 117.8) (27
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree