Modular Body Cementless Femoral Components
Michael Tanzer
Dylan Tanzer
Introduction and Rationale
Modular cementless femoral implants are now commonplace in primary total hip arthroplasty (THA). Modularity was first introduced at the head–neck junction to allow the intraoperative attachment of an aluminum oxide ceramic head on a femoral stem through a Morse-type taper (1). This modular junction allowed the surgeon to have the ability to intraoperatively adjust the leg lengths. The success of this junction, and its acceptance by surgeons, has resulted in the extension of this concept to create a modular junction in the body of various cementless femoral components used in primary THA. These implants allow the independent sizing of the proximal and distal portions of the implant to optimize fixation. As well, certain implant designs permit intraoperative adjustment of neck length, femoral offset, and version to optimally restore soft tissue tension and patient biomechanics. Modularity can also reduce the likelihood of dislocation since the version of the stem can be modified after a trial reduction, to avoid femoral neck–cup impingement.
Bone ingrowth is required for the long-term success of all cementless femoral prostheses. In order for ingrowth to reproducibly occur, the implant needs initial stability and contact with bleeding cortical host bone. These criteria can best be met when the implant is directly apposed to cortical host bone. Conventional femoral stems are designed to fit the geometry and size of the average femur. However, there is no fixed mathematical relationship between the intramedullary diameter of the femoral metaphysis and the diaphysis (2). Furthermore, this relationship varies by gender and by age (3,4,5). After examining cadaveric femora, Noble et al. (2) found that the shape of the femoral canal was more variable than most contemporary designs of femoral components could accommodate, and as a result, this would result in suboptimal contact between the femoral implant and the adjacent cortical bone. A subsequent radiographic study demonstrated that systematic differences do exist between the size and shape of male and female femora (3). Overall, the endosteal dimensions of the femora were 11% to 24% larger in the males than in females. Unlike male femora that do not change in canal shape or endosteal width as a function of age, there are profound differences in the endosteal shape and diaphyseal dimensions of the young and old female femora (3,4,5). Aging and osteoporosis in females result in significant endosteal canal widening at the isthmus, with little if any change in femoral metaphyseal width. The resultant proximal-distal femoral mismatch does not resemble the geometry of traditional femoral implants. Since conventional monoblock femoral implants assume proportional increases in neck length and metaphyseal width with increasing canal size, a traditional stem implanted into an older female with a large canal results in metaphyseal impingement, leading to proud placement of the component and resultant leg lengthening. Modularity of the body of a cementless femoral implant allows the surgeon to independently size the proximal and distal portions of the implant at the time of surgery, thereby optimizing metaphyseal and diaphyseal fit and fill. This increases the likelihood of obtaining cortical contact throughout the length of the stem, thereby maximizing stability and contact for bone ingrowth.
History of Modular Femoral Components
The first femoral component with a proximal modular sleeve was pioneered in 1967 by a Russian orthopedic surgeon, Konstantin Mitrophanovich Sivash (6,7,8). This implant was a modification of his original 1956 uncemented Sivash stem design. In 1971, the United States Surgical Corporation (Norwalk, CT) bought the license of the Sivash stem in the United States and Canada. In 1974, two of the company engineers, Douglas Noiles and Fred DeCarlo, improved the stem design by incorporating a 3-degree Morse taper, adding a distal coronal slot to avoid the potential risk of splitting the femur and adding eight distal flutes to prevent failure by rotation of the stem in the femoral canal. In 1975, the modified stem was renamed the SRN (Sivash Russin Noiles) Total Hip Prosthesis. In 1982, Douglas Noiles and Anthony Whittingham started Joint Medical Products (New Brunswick, NJ), bought the rights of the stem from United States
Surgical Corporation and renamed it the Sivash-Range of Motion (S-ROM). The stem was modified over the following 3 years to create essentially the same stem that is in use today. These modifications included having modular heads, polishing the distal stem, and adding the calcar spout and ZTT steps. In 1995, Joint Medical Products was acquired by DePuy Orthopaedics, which was subsequently acquired by Johnson & Johnson in 1998. The S-ROM prosthesis has the longest history of clinical use and remains the gold standard to which other cementless modular body femoral prostheses are compared. As a result, this chapter will deal primarily with the outcomes of the S-ROM prosthesis.
Surgical Corporation and renamed it the Sivash-Range of Motion (S-ROM). The stem was modified over the following 3 years to create essentially the same stem that is in use today. These modifications included having modular heads, polishing the distal stem, and adding the calcar spout and ZTT steps. In 1995, Joint Medical Products was acquired by DePuy Orthopaedics, which was subsequently acquired by Johnson & Johnson in 1998. The S-ROM prosthesis has the longest history of clinical use and remains the gold standard to which other cementless modular body femoral prostheses are compared. As a result, this chapter will deal primarily with the outcomes of the S-ROM prosthesis.
Implant Design
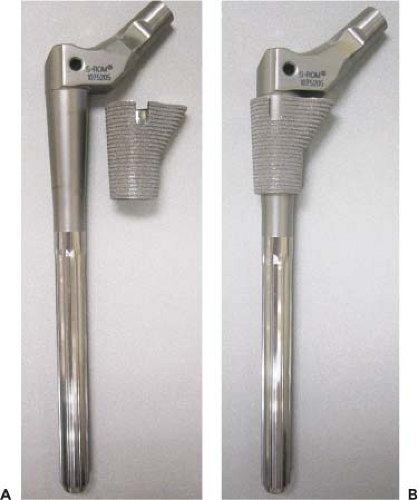
The S-ROM prosthesis (DePuy Orthopaedics Inc., Johnson & Johnson, Warsaw, IN) is made of titanium alloy and uses a Morse-type taper to join a proximal metaphyseal segment (sleeve) to the intramedullary stem portion of the implant (Fig. 58.1). It is designed to maximize the intramedullary fit proximally and distally so as to minimize micromotion and thereby enhance bone ingrowth. In a biomechanical cadaveric study, Ohl et al. (9) demonstrated that tight fixation of the S-ROM implant, both proximally and distally, provided rotational stability of 66 Nm, compared to 71 Nm in a cemented primary stem—approximately 22 Nm are required for most daily activities. The proximal sleeve is 4 cm long and is porous coated with a single layer of beads to allow bone ingrowth. The sleeve has a spout designed to fit into the metaphyseal flare of the upper femur (Fig. 58.1). This ZTT sleeve has steps to convert hoop stresses to compressive axial stresses, and its eccentric shape helps provide rotational stability. For femora without a metaphyseal flare, such as in severe developmental dysplasia of the hip (DDH), a small conical secure proximal arthropor (SPA) sleeve is also available. The stems have distal flutes that increase the diameter by 1.25 mm and are designed to enhance rotational stability. The distal stem is polished to prevent distal bony ongrowth. The surgical technique involves milling the bone rather than broaching it. First, the distal canal is reamed and then the proximal metaphysis is machined with conical reamers. A calcar reamer is used to prepare a bed for the sleeve spout, with orientation dependent upon the bony anatomy. Since the stem and sleeve have complete rotational independence, the sleeve may be impacted into the metaphysis in any degree of version. In vivo, the taper is loaded in compression, which minimizes micromotion and provides rotational stability of up to three times a patient’s body weight (10). The various stem diameters and lengths, proximal body heights and offsets, sleeve diameters and calcar spout lengths, and head lengths and diameters provide 10,398 combinations, resulting in an “off-the-shelf” custom implant (8).
Modularity Concerns
Despite its potential advantages, the appeal of modularity of the body of a femoral stem has to be tempered by the potential for corrosion, fretting, cold welding, and catastrophic fracture of the modular junction. The S-ROM stem has the longest clinical history and its taper has been studied more than any other primary hip stem with a modular body. Generally, in vivo and retrieval studies of explanted S-ROM stems have demonstrated minimal changes at the stem–sleeve interface, but catastrophic failures of the modular junction that have been reported (Fig. 58.2). Kop et al. (11) reported a retrieval study of 15 S-ROM stems revised for pain/loosening (7), infection (6), dislocation (3), and metallosis (1) at a mean of 61 months after their primary surgery. The stem–sleeve interface demonstrated no corrosion in 12 cases and only minimal corrosion in three. There were no cases of moderate or extensive corrosion of the modular interface. Some degree of fretting was noted in 67% of the cases. Bobyn et al. (12) analyzed 17 retrieved S-ROM stem–sleeve interfaces and found no cases of corrosion at a mean follow-up of 2.3 years. Small areas of surface damage from fretting were present and typically involved less than 20% of the interface and measured only a few square millimeters. Other in vitro studies have corroborated these retrieval studies, demonstrating only incomplete, small, localized fretting damage occurring at the modular body junction (12,13,14). This is in contrast to a study by Fraitzl et al. (15), who analyzed nine S-ROM stems retrieved at an average of 3 years following their primary THA. The hips were revised for loosening in three cases, infection in two, periprosthetic fracture in two, mechanical in one, and for a leg length discrepancy in one case. Severe corrosion with discoloration, delamination of the metallic surface, and black debris
occurred in two cases; five modular junctions showed moderate changes with discrete areas of burnishing surrounded by discoloration or significant amounts of black debris and two junctions showed no or minimal macroscopic evidence of surface alterations. Similar findings have been reported by Huot Carlson et al. (16), who evaluated 71 nonfractured S-ROM stems retrieved at revision surgery. Overall, fretting and corrosion were seen on 65% and 88% of stems respectively, and 42% and 86% of sleeves respectively. There was no correlation between the extent or severity of corrosion and/or fretting with stem diameter, stem offset, neck length, femoral head size, or patient demographics. Only the surgeon-determined patient activity level prior to the onset of symptoms showed a statistically significant relationship to the corrosion severity at the stem–sleeve junction.
occurred in two cases; five modular junctions showed moderate changes with discrete areas of burnishing surrounded by discoloration or significant amounts of black debris and two junctions showed no or minimal macroscopic evidence of surface alterations. Similar findings have been reported by Huot Carlson et al. (16), who evaluated 71 nonfractured S-ROM stems retrieved at revision surgery. Overall, fretting and corrosion were seen on 65% and 88% of stems respectively, and 42% and 86% of sleeves respectively. There was no correlation between the extent or severity of corrosion and/or fretting with stem diameter, stem offset, neck length, femoral head size, or patient demographics. Only the surgeon-determined patient activity level prior to the onset of symptoms showed a statistically significant relationship to the corrosion severity at the stem–sleeve junction.
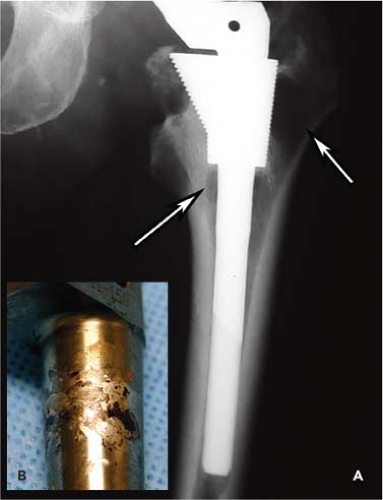
It is clear that metal degradation products released from metallic implants can have local and systemic biologic and clinical effects in the short and long term (17,18,19). The metallic debris is commonly liberated from the modular junction, rather than passive dissolution from porous ingrowth surfaces (19). The additional taper junction in a modular body femoral implant is subject to high stress levels that could, theoretically, increase metal ion and debris concentration locally and systemically. In spite of this extra junction in the body of the S-ROM femoral component, of the thousands of patients who have been reported in the literature with this stem, there are only a few reports that have described osteolysis or metallosis related to the implant. Tanzer et al. (20) reported a case of focal osteolysis adjacent to a well-ingrown proximal sleeve that was felt to be related to the body modular junction. This patient underwent an open reduction and revision for a nonconcentric reduction after dislocating his hip 7 years following a THA with the S-ROM stem. His preoperative radiographs demonstrated femoral osteolysis with no apparent polyethylene wear (Fig. 58.3A). At surgery, there was no apparent wear or oxidation of the polyethylene liner to account for the osteolysis, so the femoral stem was disengaged from the well-ingrown metaphyseal sleeve. The stem had obvious fretting in the region of the stem–sleeve taper, without obvious titanium debris in the adjacent bone or joint (Fig. 58.3B). There was no significant fretting at the head–neck junction taper. In the retrieval study by Kop et al. (11), one S-ROM prosthesis was revised for metallosis. No specifics of this case were given.
To help understand the risk of titanium debris generation by the S-ROM modular femoral component, Bono et al. (21




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree