13
Microfracture Technique
Chondral defects of the knee are frequently associated with ligamentous, meniscal and combined injuries of the athlete’s knee.1 Alone, or in conjunction with other associated intrinsic knee injuries, chondral and osteochondral defects can be responsible for pain, effusion, mechanical symptoms, and significant limitation of the athlete. Rarely do these lesions heal spontaneously, and with time most patients develop degenerative changes associated with these defects.2–4 A variety of treatment options including arthroscopic lavage, marrow stimulating or microfracture techniques, autologous chondrocyte implantation, and osteochondral implantation, allogenic and autograft, have been described with variable success.1 Unlike ligament disruption, there are no reconstructive or restorative techniques at present that restore the chondral defect to normal hyaline articular cartilage. This chapter describes the indications, patient selection, surgical technique, postoperative care, and complications associated with the microfracture technique.
 Evaluation
Evaluation
Evaluation of the patient with an articular cartilage lesion is best accomplished with a methodical approach. There are numerous published treatment algorithms,1 but understanding how best to navigate the algorithm is probably of greater importance. In 1998, the International Cartilage Repair Society made recommendations on an approach to include specific variables so that comprehensive understanding of the injury was elucidated.
A thorough history should elucidate the onset and chronicity of symptoms, describe and assess the symptoms, and determine their traumatic or spontaneous origin. A history of systemic medical problems including collagen disorders or human leukocyte antigen (HLA)-B27 associations is also essential. Previous treatment for the problem is also instrumental in the management and decision-making process.
Despite a complete physical examination, the most likely clinical presentation of full-thickness cartilage injuries are mechanical complaints consistent with a loose body or simply pain that is localizable to a specific compartment. Patients frequently have recurrent effusions, but joint line pain or a palpable point of tenderness is not always present. Ligamentous examination and assessment of overall alignment are essential.
Radiographic assessment is discussed in greater detail in earlier chapters. Standard radiographs [standing full length anteroposterior (AP), lateral, and 45-degree posteroanterior (PA) views] are used to evaluate the presence of a lesion, arthritic changes, and axial alignment. Magnetic resonance imaging (MRI) is also used to delineate the location, size, and depth of the lesion, as well as to help define associated subchondral bone bruising and meniscal and ligamentous injury. The use of radionucleotide bone scan is not as well defined, but we believe it can be a useful tool, especially in determining bony activity in previously operated knees.
 Indications
Indications
The general indication for microfracture is a full-thickness articular cartilage defect on the weight-bearing aspect of the tibia, femur, trochlear groove, or patella. Microfracture has also been used on chondral defects in the humerus, glenoid, talus, and capitellum. It is difficult to apply an age threshold; however, usually young patients with a small, contained defect are likely to improve. Microfracture is not our recommended treatment for osteoarthritis. The ideal condition for a microfracture is a symptomatic, contained, femoral condylar lesion that is less than 2 cm2 in size. We do not routinely prophylactically treat asymptomatic defects, nor do we convert partial-thickness lesions into full-thickness lesions.
We are very hesitant to perform microfracture or any articular cartilage preservation procedure in patients with axial malalignment. Sharma et al5 reported that a varus malalignment of greater than 5 degrees increases the risk of medial compartment osteoarthritis fourfold, and that valgus alignment increases the risk of lateral compartment osteoarthritis fivefold. These patients are often candidates for tibial or femoral osteotomy in a staged or sometimes simultaneous fashion. Patients with systemic diseases and inflammatory arthritides, such rheumatoid arthritis and systemic lupus erythematous, are not candidates for microfracture.
 Surgical Technique
Surgical Technique
The goal of this procedure is to penetrate the calcified cartilage layer, leave the subchondral plate intact, and penetrate the subchondral bone to create an access channel for mesenchymal stem cells to differentiate into a stable repair tissue, that is, fibrocartilage.
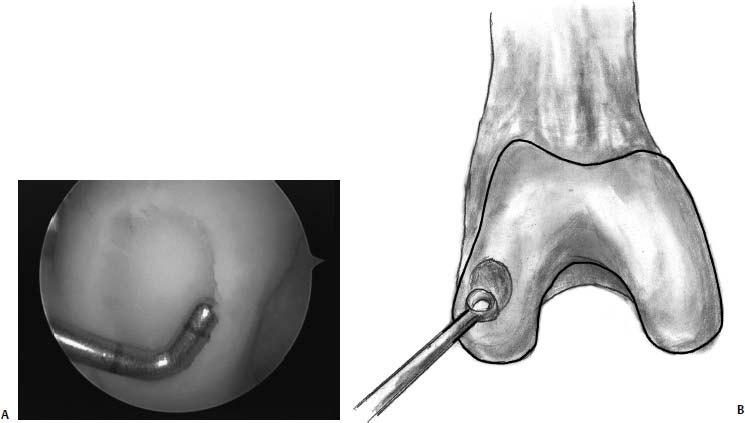
FIGURE 13–1 (A) The lesion has been debrided of loose and worn cartilage. Care is taken to remove the calcified cartilage layer and to leave a stable rim of healthy cartilage around the lesion. (B) A ring curette may be used to remove the calcified cartilage layer.
Standard arthroscopic portals are made anterolateally and anteromedially. A suprapatellar portal can also be added at the individual surgeon’s preference. We find it useful when there is copious joint debris. After completing diagnostic arthroscopy, we perform all intraarticular work including debridement, removal of any loose chondral fragments, and then microfracture. In conjunction with anterior cruciate ligament (ACL) reconstruction, we perform the microfracture first so as not to put additional stress on the new graft.
In preparation for microfracture, the lesion is identified and all loose or delaminated fragments are removed to create stable edges of articular cartilage. We do not routinely use thermal or radiofrequency energy to treat the cartilage defects. If a sclerotic base is present, abrasion chondroplasty is used in conjunction with a microfracture to create a bleeding base.
Small partial-thickness lesions are debrided of unstable fragments, and no further procedure is undertaken. Spuriously diagnosed lesions that are smaller than 2 cm2 and asymptomatic are debrided of any unstable edges and left alone. Partial-thickness lesions should not be debrided into full-thickness lesions.
The contained lesion is debrided down to exposed bone using an arthroscopic shaver. A stable rim of cartilage now surrounds the lesion (Fig. 13–1). Steadman et al4 point out that it is essential to remove the layer of calcified cartilage. This is performed with a curette, or with the shaver used in the high-speed forward mode, but not with a high-speed burr. Although it is important to create a roughened surface for a clot to adhere to, it is equally important not to remove so much subchondral bone as to compromise the structural integrity of the bone.
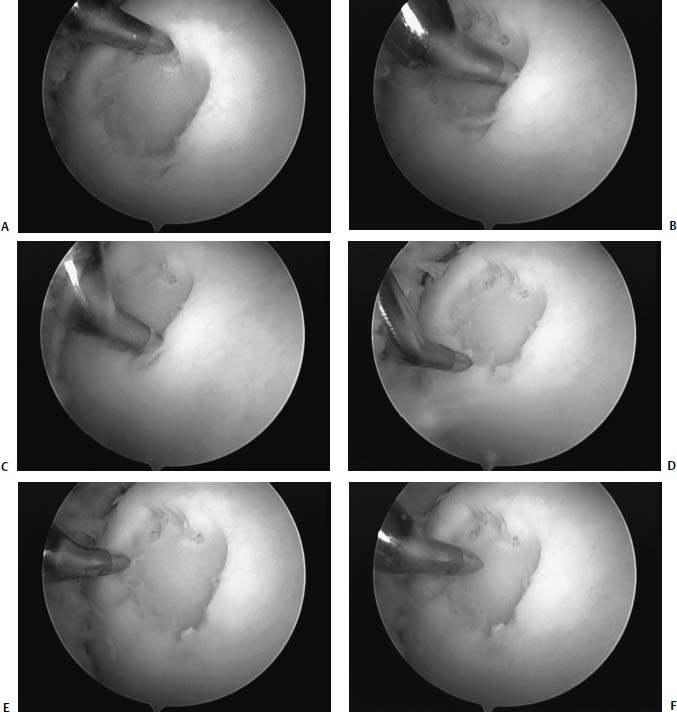
FIGURE 13–2 (A) Microfracture is performed systematically at the periphery of the lesion starting at the 12 o’clock position. (B–E) The microfracture is then carried around like the face of a clock until the 12 o’clock position is reached again. (F) Once the periphery has been microfractured, then the central portion of the lesion is addressed.
Starting peripherally in the lesion, an awl is penetrated ~4 mm into the bone, such that the subchondral bone is penetrated and either fat or blood is seen coming from the hole (Fig. 13–2). Penetration of the bone is ideally accomplished perpendicular to the articular surface. This is done with various angled awls, or more simply at different knee flexion angles. The procedure is continued around the periphery, using the awl to penetrate subchondral bone, leaving 2-mm bridges between the punctures, without causing adjacent holes to become confluent with one another. This is continued, working centrally until the defect is filled (Fig. 13–3).Satisfactory penetration of the subchondral bone may be assessed by visualization of bleeding bone or fat droplets, which is most easily obtained by creating a negative pressure (inflow off, suction on) at the end of the microfracture procedure (Fig. 13–4).
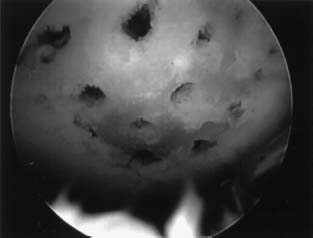
FIGURE 13–3 The defect is then completely filled, taking care not to allow the channels to coalesce, which would lead to breakdown of subchondral bone.
The high cost of awls has been criticized, and the less expensive use of Kirschner wires (K-wires) has been advocated. However, K-wires, when drilled into hard subchondral bone, can generate heat and subsequent thermal necrosis of the same tissue that is being stimulated to fill the chondral defect. The theoretical advantage of arthroscopic awls versus the risk of thermal necrosis with drilling technique is not proven, and we recommend using whichever technique surgeons are most comfortable with. Drilling with K-wires can also theoretically leave smooth channels, which “super-clot” will not adhere to, but again this has not been proven. Retrograde drilling, using the ACL guide to pinpoint the area of osteonecrosis can assist in difficult to reach areas of necrosis.
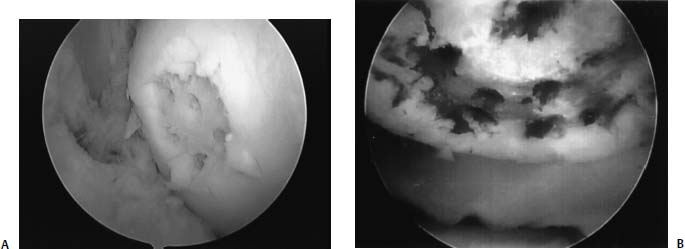
FIGURE 13–4 (A) Fat droplets (at the 4 o’clock and 5 o’clock positions). (B) Blood may be seen to extravasate from the channels.
The patellofemoral joint warrants special mention. It is paramount to keep in mind that chondromalacia patella frequently exists in the asymptomatic state, and responds to a wide number of treatments that do not address the articular surface.6 Similarly, trochlear lesions are usually well shouldered and nonprogressive. Trochlear defects are usually long and narrow. If the lesion is large enough to engage the patella and allow the patella to articulate on bare bone, the area is treated. It may be that the patellofemoral lesions are less likely to be pain sources and more likely to be particle generators. Looking for the exact etiology of the anterior knee pain can be challenging. Only when the more probable cause of anterior knee pain, such as malalignment and instability, are ruled out or treated should patellofemoral microfracture be undertaken. This is not to suggest that microfracture is not a viable option in this anatomic location; rather, we are suggesting that the surgeon proceed systematically.
 Postoperative Protocol
Postoperative Protocol
The correct rehabilitation of any surgical procedure can often be one of the most critical aspects of successful outcomes. The same principle holds true for microfracture techniques, as the proper physical environment for mesenchymal cell differentiation into stable repair tissue can be modulated by therapy.
We believe that motion after microfracture treatment is critical. The motion may simply encourage nourishment for the differentiating mesenchymal cells. It has been suggested that the motion may mediate a cellular signal for the mesenchymal cells to differentiate in articular cartilage-like cells.3 Accordingly, we are firm proponents of immediate motion. We use a continuous passive motion (CPM) device initially; however, it is discontinued as soon as uninhibited pain-free range of motion is achieved. This is routinely assessed at the first postoperative visit, 7 days after surgery. We recommend stationary cycling as an integral part of the daily postoperative protocol. Steadman et al4 are strong proponents of CPM for 8 hours per day for more than 8 weeks. This is often too cumbersome and impractical for our patient population.
In general, patients are encouraged to walk with crutches, initially non-weight bearing, and to be as mobile as possible to help limit the risk of venous thromboembolism. We do not use any pharmacologic agents to specifically decrease this risk. Treatment with ice or a cold therapy system is very important to help control postoperative swelling. We recommend ice for 20 minutes three to four times a day until swelling is essentially absent. We do not routinely use postoperative bracing, unless a ligamentous procedure has been performed. In isolated lesions, we ideally keep the patient touch-down weight bearing (the weight of the patients leg may rest comfortably on the ground) for 4 to 6 weeks.
Early range-of-motion exercises are important to emphasize. Equally important are immediate initiation of isometric quadriceps exercises and straight leg raises with ankle weights. Two weeks postoperation, aquatic exercises and swimming are encouraged. Gait retraining and strength training are initiated at 8 weeks, and return to sports is permitted when effusion is minimal or absent after exercise, and strength testing reveals 85% of the contralateral leg strength. This is usually at 4 to 6 months, depending on the size of the lesion. Maximal improvement is commonly observed as late as 2 years after the index procedure.7
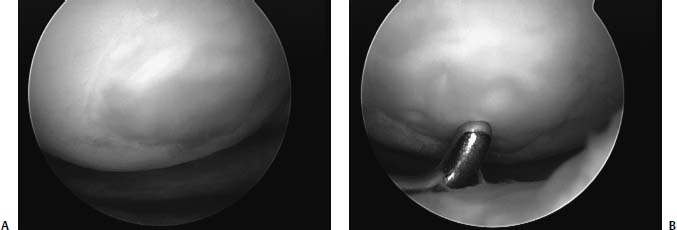
FIGURE 13–5 (A) Lesion 6 months after microfracture filled with fibrocartilage. (B) Note that the repair tissue is soft and is “dimpled” with a probe.
 Complications
Complications
The most common complications of microfracture are more specific to knee arthroscopy rather than to the microfracture technique. Certainly wound infection, pain, and persistent effusions are possible, and all are treated with conventional means. Deep venous thrombosis is an uncommon event and should be treated accordingly.
A failed microfracture, whether defined as persistent focal pain, recurrence of preoperative symptoms, or development of mechanical catching symptoms, is a recognized complication. Occasionally, repeat arthroscopy reveals a hypertrophic scar, which can be debrided to a more congruent bed. This is frequently successful. Treatment of failed microfracture lesions requires further workup and depends on the size and location. Rarely will we treat a lesion that has failed microfracture with a second microfracture procedure; rather, we consider autologous chondrocyte implantation or osteochondral auto/allografting, depending on the size of the residual defect.
 Healing
Healing
Similar to the other marrow stimulating techniques, the basic premise of microfracture is to penetrate the subchondral bone, leaving the bony architecture intact, and create an access channel to the pluripotential mesenchymal stem cells that reside within the bone marrow. The healing response of articular cartilage is well described in an earlier chapter. Simplified, marrow-stimulating techniques generate reparative tissue that is fibrocartilage (Fig. 13–5). Instead of principally type II collagen (along with types VI and IX) normally found in articular cartilage, the repair tissue is predominantly type I collagen. It is likely that the composition difference accounts for the wear characteristics and longevity of the repair.2–4
 Results
Results
Left untreated, cartilage defects rarely heal spontaneously. Messner and Maletius8 reported on the natural history of traumatically acquired full-thickness cartilage injuries in 28 athletes treated conservatively. Good or excellent results were reported in 22 of 28 patients at 14 years when left untreated. However, radiographic evidence of joint space narrowing was observed in 43% of patients, and these authors concluded that this rate of radiographic change was “twice as high as in patients with partial meniscectomy with intact cartilage at similar follow-up time.” It is important to recognize that not all chondral defects are symptomatic, and prophylactic treatment of asymptomatic lesions is at best controversial. However, patients who have symptoms that are consistent with their articular cartilage lesions probably will have the best outcome when they are treated.
To best understand the benefits of microfracture it is useful to evaluate the benefits of arthroscopic lavage and debridement. The treatment of athletes poses greater therapeutic challenges than treating a lower demand patient, who may not have the same time constraints to return to practice. As such, there may be a role for lavage. Although they have been recently criticized in the literature for the treatment of degenerative cartilage changes,9 lavage and debridement may have a role in the athlete who has symptoms from free cartilage fragments. In fact, lavage alone may have some therapeutic role. Certainly any free-floating fragments should be removed, as they may contribute to recurrent effusions or synovitis. Loose edges of articular cartilage may cause mechanical symptoms and be a source for debris. Fu et al10 examined the results of debridement for full-thickness defects and demonstrated a 55% improvement at 3-year follow-up. Hubbard’s11 results demonstrated a 59% improvement for debridement of the medial femoral condyle at 5-year follow-up, versus only 12% for patients with similar lesions treated with lavage alone. Levy et al12 independently corroborated these results with reported improvement and return to play in soccer players with lavage and debridement alone.
When a symptomatic focal chondral lesion is identified, lavage alone may be insufficient, and a marrow stimulating technique may be useful. The concept of mesenchymal stem cell stimulation was initiated over 50 years ago. In 1959, Pridie reported on open arthrotomy and drilling to treat cartilage defects.13 In 1979, Ficat et al14 introduced spongialization, in which the subchondral bone was debrided to stimulate a healing response. Sprague15 first introduced arthroscopic treatment in 1981, Jackson16 introduced abrasion arthroplasty in 1984, and Steadman and Rodrigo’s group17 introduced “ice-pick” microfracture in 1994. It is important to differentiate microfracture from the more invasive abrasion arthroplasty, where the subchondral plate is disrupted. With the exception of the “ice-pick” microfracture, most of these treatments offered the patient initial relief, but it was all short-term, as the clinical results deteriorate over time and may not be significantly different from those of debridement.1
Steadman et al18 reported on microfracture results at 3- to 5-year follow-up, and they found 75% of patients were improved, 20% unchanged, and 5% were worse. Activities of daily living (ADL) could be performed by 67% of patients. The ability to do strenuous work also improved. The specific negative predictive factors included advanced age, preoperative joint space narrowing, and isolated chondral defects. It is important to recognize that the histology of these repairs was a hybrid mixture of hyaline and fibrocartilage, and that the durability of this tissue still remains to be fully elucidated.
In a second study, Steadman and Rodrigo’s group17 reported improvement in the appearance of the articular surface following microfracture. Patients who received CPM for 6 to 8 hours a day for more than 6 weeks had better results. Furthermore, at follow-up, patients who did not receive CPM had a threefold chance of having exposed subchondral bone after microfracture.
Williams et al19 examined 34 patients, most with isolated Outerbridge grade IV lesions, who were treated with microfracture. The mean interval to follow-up was 21.1 months. They reported that the mean ADL score increased, the mean Short Form (SF-36) physical component score increased, the mean SF-36 mental component score decreased, and that none of the 34 patients required further surgery of the affected limb. The authors concluded that patients with chondral and osteochondral lesions who undergo the microfracture abrasion technique experience significant clinical improvement at an approximate 2-year follow-up interval.
The routine use of microfracture in older patients, with focal degenerative changes has previously not been recommended. However, Steadman et al20 examined the results of microfracture technique in patients 40 years of age or over (range: 40–70 years) with Outer-bridge grade IV degenerative lesions and no evidence of concomitant ligament or meniscus injury or varus/valgus malalignment. At an average of 2.5 years follow-up, all subjective parameters measured (pain, swelling, limping, walking, stair climbing, level of sport, and ADL) demonstrated significant improvement over preoperative status. Similarly, functional outcome scores demonstrated significant improvement: mean Lysholm score and mean Tegner Activity Scale score improved. Thirteen patients (15.5%) required repeat arthroscopy within 5 years of the initial microfracture procedure for lysis of adhesions. The authors concluded that the microfracture technique is an efficacious surgical option for the treatment of degenerative chondral lesions of the knee. This solitary report does not justify microfracture techniques as an option for arthritis, but perhaps pushes the indication envelope further back.
There are limited data comparing microfracture to alternative treatments. The largest prospective randomized study21 comparing autologous chondrocyte implantation (ACI) and microfracture examined 80 patients; 40 patients were treated with ACI and 40 with microfracture. The patients had symptomatic single defects on the femoral condyle between 2 and 10 cm2. They all had stable knees with no osteoarthritis. The two groups followed identical rehabilitation protocol after surgery. Follow-up examination by an independent observer was done after 12 and 24 months. There were four failures in the ACI group, defined as reoperation because of nonhealing of the primary treated defect, and two in the microfracture group. Reoperations due to trimming and debridement were done in 10 patients in the ACI group and four in the microfracture group. After 1 year, both groups had significant clinical improvement (Lysholm and visual analog scale pain score). The outcome was slightly, but not significantly, better in the microfracture group than in the ACI group after 1 year. Histologic evaluation of 67 patients was performed and graded as hyaline-like (versus hyaline-fibrocartilage or fibrous) in 71% of ACI patients as compared with only 40% of the microfracture patients. Histology was graded as fibrous in 31% of the microfracture patients, compared with 3.1% in the ACI group. This important study suggested that there was no significant clinical benefit of one treatment versus the other. The ACI group had a higher likelihood of requiring a second surgery; however, the histology in the ACI group was more like hyaline cartilage. The clinical importance of this is still yet to be determined, although we speculate that the longevity will be important for long-term outcome
Preliminary reports from a multicenter, prospective study7 also compared patient reported outcomes between patients treated with ACI and those treated with marrow stimulation techniques (MSTs) at a minimum of 3 years. They did not include patellar or tibial defects. At follow-up, ACI patients reported greater improvement in their overall Cincinnati Knee Rating Scale Score, pain scores, and swelling when compared with MST patients. Although ACI patients were characterized at the preoperative baseline by lower overall modified Cincinnati Knee Rating Scale scores, they were more likely to have had at least one prior surgery and to be receiving worker’s compensation. ACI patients reported greater functional improvements and significantly reduced symptoms at 3 to 5 years than did MST patients. The results from this prospective comparative study demonstrate that ACI may be more effective than MST for certain patients.
It has been repeatedly demonstrated that the collagen makeup of cartilage repairs does not match that of native cartilage. As such, efforts to improve the histologic characteristics of the regenerated cartilage include hybridization of microfracture and autologous chondrocytes or microfracture and a collagen matrix. Nehrer et al22 reported the results of microfracture with a collagen matrix added and ACI with a collagen matrix added. The animals treated with collagen implants showed better filling of the defects than did the untreated or only microfractured knees. Histologic analysis revealed the biggest amount of hyalinelike tissue was in the matrix augmented treatment group. Reparative tissue was predominantly fibrocartilage in the other groups. Although very preliminary, this is the only report of combined therapy in single defects, and suggests that there are potential methods to modulate the collagen expression in reparative tissues. This report may offer a glimpse into future treatment options.
 Conclusion
Conclusion
The future direction of articular cartilage repair will certainly involve a more complete understanding of the molecular biology of cartilage repair and development. Eventually the local delivery of the appropriate growth factors and signal molecules for the body to regenerate articular cartilage will become a reality, in contrast to fibrocartilage that we currently stimulate. Until that time, we will continue to utilize and improve upon the techniques discussed here. Microfracture should continue to play an appropriate role in the treatment of chondral injuries until better treatment options are elucidated.
REFERENCES
1. Mandelbaum B, Browne J, F, et al. Articular cartilage lesions of the knee. Am J Sports Med 1998;26:853–861
2. Buckwalter JS. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther 1998;28:192–202
3. Cohen NP, Foster RJ, Mow VC. Composition and dynamics of articular cartilage: Structure, function and maintaining healthy state. J Orthop Sports Phys Ther 1998;28:203–215
4. Steadman J, Rodkey W, Rodrigo J. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Rel Res 2001;391:s362–s369
5. Sharma L, Song J, Felson D, et al. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA 2001;286:188–195
6. Unverferth K, Minas T. Surgical management of isolated chondral defects. Current Opinions in Orthopedics 2002;13:1–8
7. Erggelet C, Anderson A, Arciero R et al. Marrow stimulation techniques versus autologous chondrocyte implantation for treatment of full-thickness chondral defects of the knee. Comparison of patient outcomes at 3–5 years. Proceedings of the 4th International Symposium on Cartilage Repair. June 2002, Toronto, Canada
8. Messner K, Maletius W. The long term prognosis for severe damage to weight bearing cartilage in the knee. Acta Orthop Scand 1996;67:165–168
9. Mosely J, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002;347:81–88
10. Fu F, Browne JE, Errgelet C, et al. A controlled study of autologous chondrocyte implantation versus debridement for full thickness articular cartilage lesions of the femur: results at three years. AAOS 68th Meeting Proceedings 2001, Poster No. PE 035, pp. 377–378
11. Hubbard J. Arthroscopic surgery for chondral flaps in the knee. J Bone Joint Surg Am 1987;69:794–796
12. Levy AS, Lohnes J, Sculley S. Chondral delamination of the knee in soccer players. Am J Sports Med 1996;67:165–168
13. Insall J. Intra-articular surgery for degenerative arthritis of the knee. A report of the work of the late K. H. Pridie. J Bone Joint Surg Br 1967;49:211–228
14. Ficat RP, Ficat C, Gedeon P, Toussaint JB. Spongialization: a new treatment for diseased patellae. Clin Orthop 1979;144:74–83
15. Sprague NF. Arthroscopic debridement for degenerative knee joint disease. Clin Orthop 1981;160:118–123
16. Jackson RW. Arthroscopic surgery. J Bone Joint Surg Am 1983;65: 416–420
17. Rodrigo JS, Steadman JR, Sillman JF, et al. Improvement of full thickness chondral defect in the human knee after debridement and microfracture using continuous passive motion. Am J Knee Surg 1994;7:109–116
18. Steadman JR, Rodkey WG, Singleton SB, et al. Microfracture technique for full thickness chondral defects. Technique and clinical results. Oper Tech Orthop 1997;7:300
19. Williams R, Marx R, Jones E, et al. A prospective outcome analysis of patients treated with microfracture abrasion for chondral lesions of the knee: a preliminary review. Proceedings of the 4th International Symposium on Cartilage Repair, June 2002, Toronto, Canada
20. Steadman MD Jr, Miller B, Briggs K, et al. Patient satisfaction and functional outcome after microfracture of the degenerative knee. Proceedings of the 4th International Symposium on Cartilage Repair, June 2002, Toronto, Canada
21. Knutsen G, Lars Engebretsen L, Ludvigsen T, et al. Autologous chondrocyte implantation versus microfracture. A prospective randomized Norwegian multicenter-trial. Proceedings of the 4th International Symposium on Cartilage Repair, June 2002, Toronto, Canada
22. Nehrer S, Dorotka R, Bindreiter U, et al. Repair in articular cartilage defects in a sheep model treated by microfracture and a collagen trilayer matrix. Proceedings of the 4th International Symposium on Cartilage Repair, June 2002, Toronto, Canada
< div class='tao-gold-member'>



