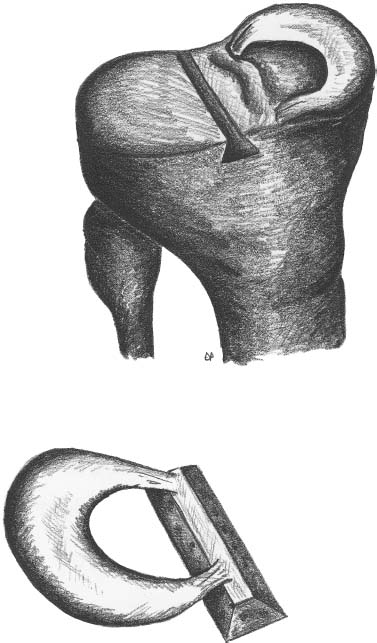
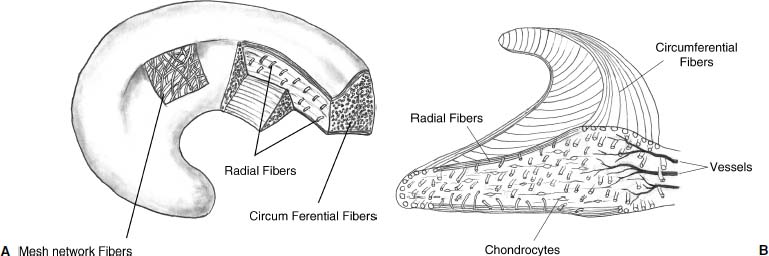
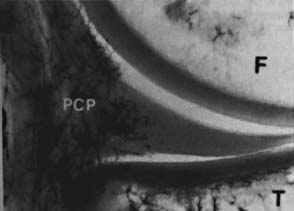
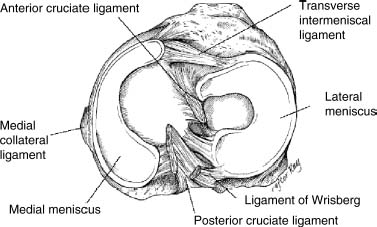
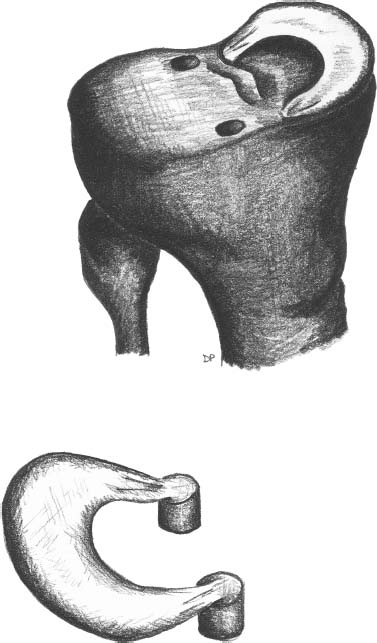
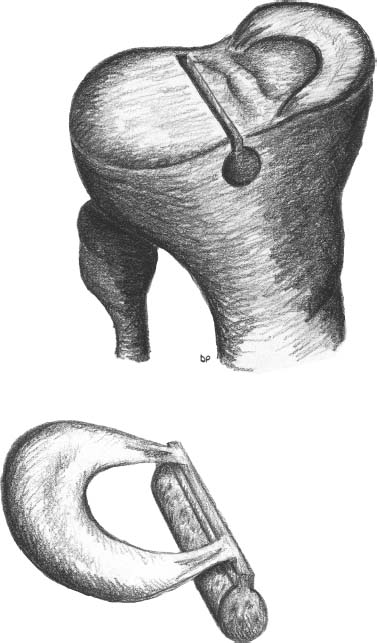
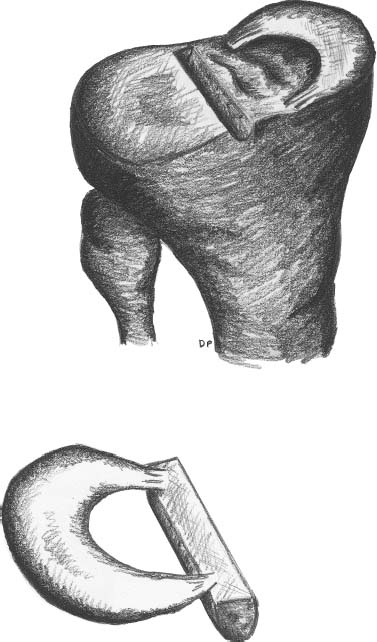
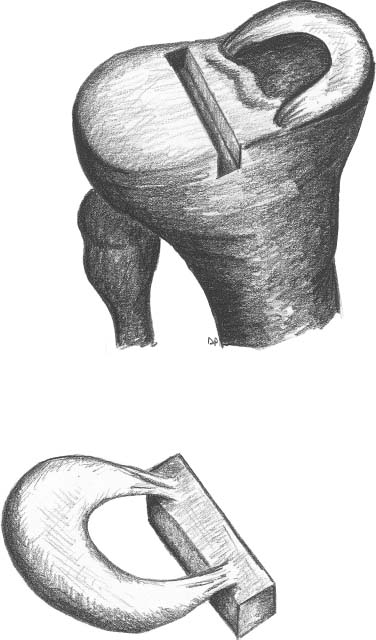
23 The meniscus was not always held in high esteem. In the early 1900s it was considered to be a functionless vestigial structure. It wasn’t until Fairbanks’s study in 1948 that the potential importance of the meniscus as a shock absorber was recognized. As more knowledge of the structure and function of the meniscus were obtained, its preservation became a prime concern. The development of arthroscopy created a procedure by which uninjured portions of the meniscus could be maintained. This ultimately led to the capability to repair the meniscus. Unfortunately, orthopaedic surgeons were confronted with patients who either had previous subtotal meniscectomies or irreparable tears requiring complete meniscectomies. The challenge was to maintain knee function in the postmeniscectomy knee. This challenge was answered by turning to the use of allograft meniscal tissue. The development of meniscal transplantation has evolved from a theoretical concept to an accepted surgical procedure that attempts to restore knee structure and function to near normal with a high degree of success. The structure, biomechanics, and function of the meniscus are well documented in the literature; however, a basic review pertinent to meniscal transplantation is provided here. The meniscus of the knee is a fibrocartilaginous structure represented by unique integration of cellular components and extracellular matrix. The cellular component, which is a minority portion, consists of structures termed fibrochondrocytes, reflecting the unique hybrid structure of fibroblasts and chondrocytes. They are responsible for the formation and maintenance of the extracellular matrix. The extracellular matrix is largely composed of collagen fibers. It is the special orientation of these fibers that provides strength to the meniscus. The majority of these fibers are circumferential in nature. Intermixed with these circumferential fibers are radial fibers that link and bond the circumferential fibers (Fig. 23–1). This unique configuration is the foundation of the strength of the meniscus and its ability to resist compressive and rotational stress forces. This natural-occurring architecture is the challenge to replicate in a synthetic meniscus. Although the majority of the functional component is type I collagen, types II, III, and V have also been identified. The vascular supply to the meniscus is via the geniculate system. The anterior portion of the meniscus and peripheral capsule are supplied by the medial and lateral branches of the inferior and superior geniculates. The posterior portion is supplied by the middle geniculates. The vessel branch ultimately forms a perimeniscal capillary complex that then supplies the peripheral aspect of the menisci (Fig. 23–1B and 23–2). On the medial side, the peripheral 10 to 30% of the meniscus is vascularized, whereas on the lateral aspect the peripheral 10 to 25% is vascularized. However, in children, up to 50% of the peripheral aspect of both menisci can be vascularized. Grossly the menisci are semilunar in shape, being triangular in cross section with the thickened base oriented peripherally. The medial meniscus is longer in the anteroposterior direction (Fig. 23–3). An important factor when performing medial meniscal transplantation is that the anterior horn is in line with the medial tibial eminence, posterior to the fat pad and edge of the patellar tendon. The anterior fibers of the meniscus ultimately contribute to the formation of the transverse intermeniscal ligament. The attachment of the anterior horn may also go across the anterior slope of the tibia. The lateral meniscus is more circular in shape with a smaller anteroposterior length. The position of the anterior horn is anterior to the tibial spine and anterior cruciate ligament footprint, and must be replicated when performing a lateral meniscal transplant (Fig. 23–3). The lateral meniscal anatomy is further complicated by the presence of the popliteal hiatus and the meniscal femoral ligaments. FIGURE 23–1 (A) The meniscus is composed of circumferential and radial fibers, which are held together by meshed network of fibers. (From Bullough PG, Munuera L, Murphy J, Weinstein AM. The strength of the menisci of the knee as it relates to their fine structure. J Bone Joint Surg Br 1970; 52:564–567, with permission.) (B) The circumferential fibers are in the periphery, whereas the radial fibers extend from the circumferential fibers toward the central portion of the meniscus. FIGURE 23–2 The perimeniscal capillary complex supplies the peripheral third of the meniscus. Also known as the “redred” zone. (From Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med 1982;10:90–95, with permission.) All these complexities allow the meniscus to achieve its function: load bearing, force distribution, joint stability, lubrication, and proprioception. The microstructure of the meniscus is an important component in meniscal function as the collagen forms circumferential fibers that are linked by regularly oriented collagen fibers, as previously noted. It is this network of fibers that helps to resist tension, compression, and shear and ultimately contributes to the ability of the meniscus to bear load and distribute forces. The circumferential fibers run from bony horn attachment to bony horn attachment and resist the axial load and compression loads, converting radial forces to tension forces, termed hoop stresses. As stresses can be defined as force per unit area, it is then apparent that the meniscus can reduce stress to the articular cartilage by both redirecting the forces through the hoop stress and increasing the surface contact area. FIGURE 23–3 Topography of the proximal tibia showing the ligaments and menisci in their relevant relationships. (From Pagnani MJ, Warren RF, Arnoczky SP, Wickiewicz TL. Anatomy of the knee. In: Nicholas JA, Hershman EB, eds. The Lower Extremity and Spine in Sports Medicine, 2nd ed. St Louis:Mosby, 1995:581–614, with permission.) The ability to utilize meniscal allograft as successful substitutes for the normal meniscus is founded in basic science considerations. The major factors that should be considered are immunology, chondroprotective potential, healing potential, and tissue preservation. The relatively acellular nature of the meniscus allows it to be described as immunologically privileged.1 Although the largest population of histocompatibility antigens are on the cellular components, the relatively low population and shielding by the extracellular matrix protects the tissue. Therefore, systemic rejection rarely occurs. However, it has been demonstrated that some form of immune response does occur.2 The potential for a localized immune response exists: (1) even after deep freezing, histocompatibility antigens can be expressed on the cells of a meniscal allograft; (2) the bone and synovial attachments to the meniscal allograft will remain antigenic; and (3) the existence of immunoreactive cells in fresh frozen meniscal allograft has been demonstrated.3 This localized response may have an affect on graft revascularization, repopulation, incorporation, and overall healing response. However, it appears that clinical results have not been compromised by this localized immune response. It is generally accepted, based on multiple studies, that the meniscal allograft will heal, gradually repopulate with host cells, and not be systemically rejected.4 One of the major concerns about meniscal transplantation is the chondroprotective potential, both short and long term. Several animal studies have demonstrated chondroprotective benefits of the meniscal allograft when compared with a total meniscectomy.5 However, the studies did not demonstrate results identical to a normal meniscus. Similar improvements in contact/stress in a cadaveric model have also been demonstrated. These protective benefits are short term, and animal studies demonstrating long-term chondral protection are lacking. Meniscal allograft tissue should only be obtained from a tissue-banking facility that is in compliance with the criteria established by the American Association of Tissue Banks. This standardization maximizes the quality of the tissue (no evidence of degenerative change) and minimizes the risk of disease transmission. At the present time, meniscal allograft tissue is processed by one of four techniques: fresh, cryopreserved, fresh frozen, or freeze dried. Fresh allograft tissue offers advantages regarding cell viability, but is not practical. Freeze dried tissue is associated with cell death. However, the major disadvantage of this process is the ultimate affect it has on the structural properties of the meniscal allograft, including graft shrinkage and potential damage to the collagen fibers. The processes of cryopreservation and fresh frozen tissue avoid the structural damage seen with freeze drying. Cryopreservation involves a slow freezing rate of the allograft tissue by utilizing a chemical cryoprotectant such as dimethylsulfoxide (DMSO). This method allows for a slight increase in cell viability and preservation of some degree of cell membrane integrity. The processes of cryopreservation and fresh frozen tissue both allow for prolonged storage. These processes of prolonged storage facilitate not only complete serologic testing, but also creation of a tissue bank of various subtle sizes, potentially improving tissue matching. Meniscal allograft tissue that undergoes fresh frozen processes undergoes a more rapid freezing process that results in slightly increased cell death, but without adversely affecting the structural properties of the graft. Cell viability, however, may not be an important issue, as has been shown by experimental studies that have suggested that there are no significant outcome differences between cryopreserved and deep frozen grafts. Indeed it has been demonstrated that the repopulation of meniscal allograft tissue by host-derived cells is extremely common and occurs within the first year of implantation. Future research will provide insight into the enhancement of the process of repopulation and incorporation. At the present time the utilization of fresh frozen tissue, compared with either fresh or cryopreserved, is simpler, less costly, and still provides the essential biologic scaffolding for successful function and incorporation. The general indications for meniscal transplantation are a history of documented total or subtotal meniscectomy with pain and/or disability relating to the affected compartment of the knee. However, other significant variables need to be considered. The duration of time since meniscectomy is important, especially as it relates to articular cartilage wear and possible alignment issues. The patient’s history should be correlated with the patient’s symptoms and physical examination. As part of the general knee examination, attention should be paid to the nature of the previous surgical scars, the palpation of osteophytes, ligamentous laxity, standing alignment, and the observation of gait. Radiographic evaluation should include at least the following: anteroposterior (AP) standing x-rays of both knees in full extension; non—weight-bearing, 45-degree flexion laterals; 45-degree flexion weight-bearing [posteroanterior (PA)]; and an axial view of the patellofemoral joint. Although magnetic resonance imaging (MRI) is not an imperative radiologic test, additional important information can be obtained regarding the status of the articular cartilage and the biologic activity of the subchondral bone, especially when performed with gadolinium enhancement. The ideal indications for meniscal transplantation are a symptomatic patient with (1) documented previous subtotal meniscectomy or absence of the major weight-bearing portion of the meniscus, (2) pain in the involved knee compartment, (3) less than grade 3 or correctable articular cartilage damage, (4) ligamentous stability or correctable stability, and (5) normal or correctable alignment.6,7 However, these indications will continue to evolve as the concept of cartilage restoration of the knee advances. Although the chronologic age most often referred to as a limit is 50 years, age is in reality a relative consideration. It is important to assess the biologic age, taking into consideration the environment of the knee, activity level, and ability to be compliant with the postoperative course and rehabilitation. There are several contraindications to meniscal transplantation: degenerative arthritis, inflammatory arthritis, crystal deposition disease, and active infection. It is important to determine whether the articular cartilage changes that exist are secondary to the previous meniscectomy and can be restored or whether this truly represents advanced osteoarthritic changes. Obesity has the potential to compromise a normal knee joint. Based on articular cartilage-related studies, a body mass index (BMI) of over 35 should be considered a relative contraindication. The patient who has recently undergone a subtotal meniscectomy and has not yet developed symptoms poses a significant challenge. The treatment and timing of treatment is certainly debatable as well as controversial. The natural history of the meniscal deficient knee is well documented. Nevertheless, not every postmeniscectomy patient develops degenerative arthrosis. There are several factors that are important in this treatment algorithm. Certainly these patients must undergo rigorous education about the signs and symptoms associated with knee degeneration. They should also be followed closely with standing 45-degree PA radiographs. Currently the measurement of articular cartilage degradation products is changing from a laboratory setting to the clinical realm, and may play an added role in early detection of postmeniscectomy articular cartilage damage. However, is that enough? The breakdown of articular cartilage can progress very rapidly in the meniscal-deficient joint. The factors that need to be considered are activity level, physiologic age, rehabilitative potential, and goals and expectations. In general, in the select postmeniscectomy individuals who have higher demands on their knee, rehabilitation potential, and realistic goals, there is a role for early consideration of meniscal allograft transplantation. The potential for articular cartilage breakdown in the younger patient and creation of a negative knee environment is too great to ignore. By the time the patient is symptomatic and has positive x-rays or a positive bone scan, the articular cartilage has already been damaged. When the patient has been selected for meniscal transplantation by meeting the previous described criteria, the meniscus must be obtained. Prior to ordering the meniscus, sizing studies must be performed. The most commonly accepted method utilizes standard AP and lateral radiographs with magnification markers as described by Pollard et al.8 It is important for both the surgeon and the tissue bank to measure these films and for the surgeon to comment on any special considerations that could alter accurate measurements, such as an osteophyte or previous trauma. It is also imperative to be certain of which side (right versus left) and which compartment (medial versus lateral) is being requested. This should be double-checked on the day of surgery prior to the patient’s being anesthetized and prior to defrosting of the tissue. The evolution of meniscal transplantation has allowed the procedure to advance to an arthroscopically assisted procedure with a miniarthrotomy. It may also be performed as an open procedure, especially when done concomitantly with a major articular cartilage restoration. Several different techniques have been developed for implantation. These include the double bone plug technique and the bone bridge techniques—keyhole, slot, trough, and dovetail. Regardless of which technique is selected, the essential foundation of successful meniscal transplantation is anatomic reduction and stable fixation of the meniscal allograft. The fixation of the meniscal allograft is accomplished by the bony attachments and stabilization of the peripheral rim. Most recently developed all-inside meniscal repair devices are not well adapted for meniscal transplantation fixation. The majority of these devices are designed for meniscus-to-meniscus repair, not for meniscus to capsular soft tissue. In the situation where an adequate meniscal rim remains, the use of these devices may be considered. The most reliable method of fixation is by inside-out suture fixation. The double bone plug technique9,10 was developed for transplantation of the medial meniscus and represented the first bone-anchored procedure. The bone plug technique involved the creation of bone tunnels at the site of the anterior and posterior horn attachments of the meniscus (Fig. 23–4). As compared with the medial meniscus, the anterior and posterior horn of the lateral menisci are close enough that tibial tunnel creation and subsequent fixation could be compromised. Therefore, this technique is not indicated for lateral meniscal transplant. The medial meniscus is prepared by the creation of bone plugs at the anterior and posterior horn attachments of the allograft tissue. These bone plugs are then placed into the appropriately corresponding anterior and posterior tunnels. Placement of the posterior bone plug can often be difficult and can result in fracture of the bone plug, ultimately compromising fixation. As discussed earlier, it is imperative for anatomic reduction. Therefore, it must be remembered that the anterior horn attachment is usually on the anterior slope of the tibia. Therefore, creation of a true bony tunnel may not be possible and the anterior plug may be placed more as a press-fit. To compromise this anterior placement by simply placing the anterior horn where it lies best is certainly easier, but is unacceptable. This nonanatomic placement would compromise any chance of replicating normal biomechanical function11 by creating abnormal stress and not establishing normal contact pressures. Although the use of this technique can be successful, careful attention must be given to maintaining the anatomic reduction to optimize function. FIGURE 23–4 Bone plug technique. Note that although in this illustration, a bone plug is being used for a lateral meniscal allograft, this technique is recommended only for medial meniscal transplantation. See text for explanation. The utilization of a bone bridge between the anterior and posterior horn allows for maintenance of the normal anatomic alignment of the attachment points and for superior bone fixation along the bridge. Several different bone bridge techniques have been developed: keyhole, slot, trough, and dovetail.10,12 The keyhole technique (Fig. 23–5) was developed for placement of the lateral meniscal allograft. This technique involves the creation of a keyhole in the tibial plateau with subsequent creation of a key bridge corresponding to this tunnel. The procedure can be very demanding to perform because it requires fine-tuning of the key bridge to fit the slope and shape of the keyhole. In addition, the creation of the key bridge may be compromised by the initial harvest and preparation of the meniscal allograft. The dovetail (Fig. 23–6), slot (Fig. 23–7),7 and trough techniques (Fig. 23–8) have some basic similarities. They all utilize guide systems to create a trapezoidal or rectangular-shaped bone tunnel on the tibial plateau in an anteroposterior direction. This tunnel corresponds to the anatomic position of the anterior and posterior horn attachments. Both techniques can be used on either the medial or lateral side. The greatest difference in these two techniques is in the sizing of the bone bridge. For the trough technique, the bone bridge is created so that it is press-fit in the trough tunnel. The dovetail technique also creates a press-fit tunnel that allows for stabilization so that it will not ride up in the slot. The concept of press-fit is good only if there is extremely accurate sizing and placement. These two factors are extremely important for appropriate function. The actual placement of a press-fit plug can also be difficult, and if impaction is utilized it has the potential for fracture of the bone bridge or damage to the anterior horn attachment site. In addition, once engaged, if removal of the bone bridge were required this could be extremely complicated. FIGURE 23–5 Keyhole technique. The slot technique utilizes a slightly undersized graft, which allows for positioning and fixation to be considered independently. Once the bone bridge and meniscus tissue are reduced, the knee is cycled near extension, allowing the femoral condyle to capture the meniscus and fine tune the position of the bridge. At this point it is then fixated. This allows for the construct to be fixed in a position that provides appropriate biomechanical alignment. The bone bridge can also be placed without impaction and removed for adjustment easily. The bone bridges can be fixated by suture, suture anchor, press-fit, interference fit with screws or bone dowels, or bone graft. Meniscal transplantation is a complex multifactorial biologic process. Complications can range from the standardized risks associated with arthroscopic surgery and meniscal repair to failure of the procedure. With careful preoperative planning, intraoperative attention to detail, and postoperative monitoring and compliance, these potential complications and secondary morbidity can be minimized. The goal of the procedure is to restore a functional meniscus to the knee. Anything that will create suboptimal solutions is not acceptable. The allograft must be checked for sidedness and appropriate compartment. Sizing can also be evaluated prior to initiating surgery. Unfortunately, defects in the actual tissue may not be realized until the tissue is thawed. FIGURE 23–6 Dovetail technique. Again, it is extremely important to make certain the bone bridge is adequately seeded and the meniscus accurately fixed and aligned. The compromise of either factor will lead to early postoperative complications with long-term sequelae. Postoperative infection can occur and should be addressed appropriately based on the type of organism. Arthrofibrosis can be a common complication due to the extensive nature of this surgery, especially if additional procedures are performed. It is important to recognize the development of extensive adhesions early and treat them aggressively by debridement and manipulation. FIGURE 23–7 Slot technique. FIGURE 23–8 Trough technique. The goal of rehabilitation is to allow early return to pain-free normal function without compromising the healing and remottling process. Rehabilitation from meniscal transplantation has had significant variability and evolution. Protocols have ranged from no motion to early motion, and non—weight-bearing to weightbearing as tolerated. It is important to recognize the need to protect the articular cartilage with early range of motion and mild loading to maintain the optimal matrix composition. Initially weight bearing is limited to partial weight bearing for the first 4 weeks. After 4 weeks, weight bearing may be progressed to full weight bearing as tolerated. This progression corresponds to the increased metabolic activities of the meniscal cells and their ability to withstand stress. The requirement for loading and the positive influence and remodeling process then become a positive factor. Range of motion is initially protected in a range-of-motion brace from full extension to 60 degrees of flexion for the first 2 weeks. After week 2 through week 4, flexion is increased to 90 degrees, and after 4 weeks there is progression to a full range of motion. Standard knee rehabilitation is then initiated including strengthening, proprioception, and functional training. However, squatting should be avoided. The primary goals of range of motion, strengthening, and pain relief should be accomplished before progressing to more advanced activities. The full return to unrestricted activities requires 5 to 6 months. Although the basic concept of meniscal transplantation is similar to that originated in 1984, the basic and clinical science has undergone continuous improvement regarding understanding, application, and performance of the surgical procedure. When analyzing and comparing the results of any procedure, it is important to have minimal variables between the individual series. Unfortunately for meniscal transplantation, this is rarely the case. Since Milachowski et al’s13 first publication in 1989, multiple series have been reported. However, there are different variables in each study, such as patient population, knee arthrosis, graft preservation, surgical techniques, additional surgical procedures, rehabilitation, and parameters of follow-up. Therefore, it becomes challenging and difficult to make accurate conclusions about the role of meniscal transplantation. Though Milachowski et al were the first to perform a modern-type isolated meniscal transplant in 1984, their subsequent publication in 1989 on 22 patients with an average 14-month follow-up demonstrated healing of 15 to 18 (83%) grafts peripherally by arthroscopic evaluation. Milachowski et al had used both fresh frozen and freeze dried grafts, with the fresh frozen appearing more normal microscopically. Garrett14 in 1993 reported on the 7-year follow-up of 43 patients. The patient population in this study was certainly complex (salvage-type cases). Only six patients had an isolated meniscal transplant, and 28 patients underwent second-look arthroscopies, with 20 patients (71%) demonstrating healing of the meniscal allograft. The eight failures were attributed to grade 4 chondromalacia. The remaining 15 patients were asymptomatic and considered to have a good outcome. Noyes and Barber-Westin15 evaluated a large series of patients in 1995. This series reviewed 96 fresh frozen irradiated grafts in 82 patients. The results were extremely poor. Of the total 96 implants, 58% failed completely, 31% were partially healed, and 9% healed. Twenty-nine menisci had to be removed less than 2 years after surgery. The surgical technique involved attachment only of the posterior horn with bone plugs. No transplant was attached with bone both anteriorly and posteriorly. All grafts were irradiated. The presence of grade IV chondrosis was associated with 50% failure rate. Cameron and Seha16 reported their results on 67 irradiated menisci without bone anchors in patients with generally advanced unicompartmental arthritis. These patients also underwent an osteotomy to unload that compartment at the time of surgery. At an average of 31 months’ follow-up, 87% demonstrated a good or excellent result. Van Arkel and deBoer17 reported on 23 patients with a minimum of 2-year follow-up who underwent isolated meniscal transplantation. There were three failures at less than 24 months with uncorrected alignment. The remaining 20 patients (87%) were felt to have a satisfactory result. Stollsteimer et al18 reported a series of 22 patients with 23 cryopreserved allografts. Implantation was done arthroscopically assisted with bone plugs. The patients were followed for an average of 40 months. The most significant finding in this study was pain reduction in all patients. Although not associated with an adverse outcome, the allograft demonstrated a 37% shrinkage on MRI studies. Carter19 reported on 46 transplants with a minimum 2-year follow-up. Thirty-eight of the transplants underwent arthroscopic second looks between 3 and 48 months, with most occurring at 6 months. At the time of arthroscopy, four patients were considered failures, four had visible shrinkage, and two showed signs of progression of arthritis. Thirty-two of the 38 patients (84%) reported relief of pain and improvement in activities. In 1999, Cole and Harner20 reported the results of 22 fresh frozen menisci, and 95.5% of the patients reported improvement of pain, with associated improvement in knee function in all but one case. Rodeo et al12 represented a comprehensive review of the literature at a national orthopedic meeting. In their review, as of March 2001, a total of 1599 meniscal transplants were performed in 1551 patients. Of these, direct objective evaluation of the transplanted tissue was done in only 366 menisci in 338 patients. Ages ranged from 10 to 68 and averaged 33. The series included fresh, fresh frozen, cryopreserved, freeze dried, irradiated, and nonirradiated menisci. The meniscus was implanted both with and without bone plugs. Concomitant procedures were performed in all but 136 cases. The degree of arthrosis in the knee varied from grade I to grade IV, and there was no consistent rehabilitation. The author’s personal experience (unpublished data) began in 1991. The current series consists of 94 meniscal transplants in 92 patients. During the time of this series there have been subtle changes in the surgical procedure, rehabilitation, and understanding of the associated basic science. Of these 92 patients, 36 had undergone concomitant procedures consisting of anterior cruciate ligament reconstruction, autologous cultured chondrocyte implantation, or tibial osteotomy. Sixty patients have been followed for greater than 2 years. Forty-seven (78%) of these patients can be considered to have a good or excellent result with improvement in pain and function. Nine patients would be considered failures, with the remaining patients having a fair result. The nine failures, seven of which were greater than 5 years postimplantation, were noted to have preexisting grade IV arthritic changes or uncorrected malalignment and were performed early in the series before these findings were known to be deleterious to the outcome of meniscal transplantation. The present indications, techniques, and graft selection are based on the results of these earlier studies. Certain principles have achieved somewhat uniform acceptance. The grafts should be cryopreserved or fresh frozen. The procedure may be performed either open or arthroscopically assisted with the use of bone fixation, both anteriorly and posteriorly. The patient should have a subtotal meniscectomy with normal or correctable alignment, a ligamentously stable or correctable joint, and grade II changes or less chondrosis or restorable chondral defects in the affected compartment. Ligamentous Reconstruction The requirement for ligamentous stability of the knee joint is very important. If the knee is clinically unstable, the transplanted meniscus will be subjected to the same abnormal shear forces that often result in a native meniscal tear. This same philosophy applies to all ligamentous structures of the knee joint, whether it be the anterior or posterior cruciate ligaments, the collateral ligaments, or the posterolateral corner. Ligamentous reconstruction and meniscal transplantation may be done as a single-stage procedure, and it is not necessary to compromise placement of the cruciate ligament tunnel or of the bone slot when performing an anterior cruciate ligament procedure concomitantly. The bone bridge slot technique facilitates accurate placement of the bone trough with only minimal adjustment of the tibial tunnel and without compromise of the anatomic insertional point. If there is intersection between the tunnel and the slot, the bone of the allograft bone bridge and tendon of the anterior cruciate ligament graft will be in contact. This does not compromise the fixation, placement, or function of either graft in tissue. Malalignment The importance of alignment has been previously underestimated by many. Weight-bearing x-rays must be taken as part of the preoperative evaluation. If there is malalignment of even a few degrees of the affected extremity as compared with the normal extremity, realignment osteotomy should be considered. This is especially true on the medial side, which has greater stress even in normally aligned extremities. With newer techniques allowing for more distally placed osteotomies, intersection with the tunnels or slot to the meniscal allograft can be avoided with planning. This procedure can be done as a single-stage surgery. It should be remembered that the realignment procedure is not going to be similar to that done for an osteoarthritic knee. Chondral Defects The presence of a chondral defect has previously been a contraindication for meniscal transplantation in light of the study in which Noyes demonstrated poor results in knees with grade III to IV chondrosis. With the ability to restore articular cartilage by either osteochondral grafting or autologous chondrocyte implantation, the number of treatable patients can be expanded. The choice for articular cartilage restoration should follow guidelines based on size and location as highlighted in different publications. Regardless of the technique applied for articular cartilage restoration, the meniscal allograft should be placed and sutured prior to the full osteochondral plug placement or periosteal fixation. This will avoid damage to the graft or periosteum during reduction of the meniscus and suturing of the meniscal allograft peripherally. The author’s experience with combined autologous chondrocyte implantation and meniscal transplantation has been very positive. This has resulted in the restoration of knee function and return to function in the majority of patients with minimal complications. Meniscal transplantation has evolved as a successful procedure for the reconstruction of the meniscal deficient knee. This success will be further enhanced and improved by collaboration with basic science research. Although early results were promising, with the development and understanding of the technique, more recent studies have demonstrated improved results. As meniscal repair and preservation techniques continue to advance, the meniscal deficient knee will become less common. However, the technology of meniscal transplant will continue to be an integral procedure for the preservation of knee function. REFERENCES 1. Arnoczky SP, Milachowski KA. Meniscal allografts: where do we stand? In: Ewing JW, ed. Articular Cartilage and Knee Joint Function: Basic Science and Arthroscopy. New York: Raven: 1990:129–136 2. Ochi M, Ishida O, Daisaku H, Ikuta Y, Akiyama M. Immune response to fresh meniscal allografts in mice J Surg Res 1995;58: 478–484 3. Rodeo SA, Seneviratne A, Suzuki K, Felker K, Wichiewicz TL, Warren RF. Histological analysis of human meniscal allografts. A preliminary report. J Bone Joint Surg Am 2000;82:1071–1082 4. Jackson DW, McDevitt CA, Simon TM, et al. Meniscal transplantation using fresh and cryopreserved allografts. An experimental study in goats. Am J Sports Med 1992;20:644–656 5. Szomor ZL, Martin RE, Bonar F, Murrell GA. The protective effects of meniscal transplantation on cartilage. An experimental study in sheep. J Bone Joint Surg Am 2000;82:80–88 6. Gersoff WK. Combined meniscal allograft transplantation and autologous chondrocyte implantation. Oper Tech Sports Med 2002; 10:165–167 7. Furr, J, Gersoff, WK. Current meniscal transplantation. Sports Med Arthrosc Rev 2004;12:69–82 8. Pollard ME, Knag Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy 1995;11:684–687 9. Shelton WR, Dukes AD. Meniscus replacement with bone anchors. A surgical technique. Arthroscopy 1994;10:324–327 10. Cole BJ, Carter TR, Rodeo SA. Allograft meniscal transplantation background, techniques, and results.J Bone Joint Surg 2002;84-A:1236–1250 11. Paletta GA Jr, Manning T, Snell E, Parker R, Bergfeld J. The effect of allograft meniscal replacement on intraarticular contact area and pressures in the human knee. A biomechanical study. Am J Sports Med 1997;25:692–698 12. Rodeo SA, Senevirate A, Suziki K, Felker K, Wickiewicz TL, Warren RF. Histological analysis of human meniscal allografts. A preliminary report. J Bone Joint Surg Am 2000;82: 1071–1082 13. Milachowski KA, Weismeier K, Wirth CJ. Homologous meniscus transplantation. Experimental and clinical results. Int Orthop 1989;13:1–11 14. Garrett JC. Meniscal transplantation: a review of 43 cases with two- to seven-year follow-up. Sports Med Arthrosc Rev 1993;1:164–167 15. Noyes FR, Barber-Westin SD. Irradiated meniscus allografts in the human knee. A two- to five-year follow-up study Orthop Trans 1995;19:417 16. Cameron JC, Seha S. Meniscal allograft transplantation for unicompartmental arthritis of the knee. Clin Orthop 1997;33:164–171 17. Van Arkel ER, de Boer HH. Human meniscal transplantation. Preliminary results at 2- to 5-year follow-up. J Bone Joint Surg Br 1995;77:589–595 18. Stollsteimer GT, Shelton WR, Dukes A, Bomboy AL. Meniscal allograft transplantation. A one- to five-year follow-up of 22 patients. Arthroscopy 2000;16:343–347 19. Carter TR. Meniscal allograft transplantation. Sports Med Arthrosc Rev 1999;7:51–62 20. Cole BJ, Harner CD. Degenerative arthritis of the knee in active patients: evaluation and management. J Am Acad Orthop Surg 1999;7:389–402
Meniscal Transplantation
 Anatomy and Physiology of the Meniscus
Anatomy and Physiology of the Meniscus
 Basic Science Considerations
Basic Science Considerations
 Allograft Selection
Allograft Selection
 Patient Selection and Indication
Patient Selection and Indication
 Surgical Technique
Surgical Technique
 Rehabilitation
Rehabilitation
 Results
Results
 Concomitant Procedures
Concomitant Procedures
 Conclusion
Conclusion
< div class='tao-gold-member'>
Meniscal Transplantation
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree