Meningococcal Infections
C. Mary Healy
Morven S. Edwards
Carol J. Baker
Great strides have been made in our understanding of the meningococcus since the initial descriptions of “epidemic cerebrospinal meningitis” by Vieusseux in 1805, and of “petechial or spotted fever” the following year by Elisha North. More than a century ago, the causative organism, then called Diplococcus intracellularis meningitidis, was described by Weichselbaum, who observed that it was found almost exclusively within neutrophils, and that it could be differentiated morphologically from the pneumococcus.
The introduction of serum therapy in 1907, the later addition of sulfonamides and penicillin in the 1940s, and the current availability of intensive care support have dramatically improved the outcome of these infections. However, Neisseria meningitidis causes fulminant infections that are fatal within hours after the onset of symptoms, and Herrick’s statement, in 1919, that “no other infection so quickly slays” still holds true. Many problems must be resolved before meningococcal disease is successfully eradicated.
MICROBIOLOGY
Neisseria is a member of the family Neisseriaceae, which also contains the genera Kingella, Eikenella, Simonsiella, and Alysiella. N. meningitidis is differentiated from N. gonorrhoeae and less pathogenic Neisseria species on the basis of carbohydrate fermentation reactions. N. gonorrhoeae ferments only glucose, but most strains of N. meningitidis ferment glucose and maltose. Laboratory identification of the few strains that fail to produce acid from either carbohydrate may be particularly difficult.
Meningococci are fastidious. For optimal growth, they require enriched media, such as chocolate, blood, or Mueller-Hinton agar, and a 3% to 10% CO2 atmosphere. Colonies are 1 to 5 mm in diameter, translucent, and nonhemolytic. N. meningitidis is a gram-negative, biscuit-shaped or coffee bean-shaped diplococcus with rounded outer and flattened inner margins; it has a polysaccharide capsule to protect it from phagocytosis. Specific capsular polysaccharide antigens allow the classification of meningococci into at least 13 serogroups. Serogroups A, B, C, Y, and W135 are responsible for human disease. The other groups are designated D, X, Z, 29-E, H, I, K, and L. The principal noncapsular cell-wall antigens include lipooligosaccharide, which is analogous to the lipopolysaccharide of enteric gram-negative bacilli, and the outer membrane protein (OMP). At least 12 different lipooligosaccharide serotypes exist. Groups B and C can be subdivided into at least 15 protein types on the basis of antigenically distinct OMPs. The OMPs allow the organism acquire iron (required for growth) by binding to host heme, transferrin, and lactoferrin, and are part of a lipoprotein-lipopolysaccharide complex that is responsible for the endotoxinlike effect observed in invasive infection.
Meningococci also contain pili or fimbriae that enhance attachment to nasopharyngeal epithelial cells, thus allowing colonization and invasion.
EPIDEMIOLOGY
Meningococcal infection is primarily a disease of childhood. In general, an inverse relation exists between age and attack rate, with the exception of an increased incidence among 15- to 24-year-olds who account for up to 20% of meningococcal-related deaths. More than 50% of the patients are younger than 4 years, and the highest age-specific attack rate is found among infants less than 12 months of age. Infection has been described during the first month of life. Among children, the genders are affected equally. Outside of infancy, persons aged 16 to 18 years and college freshmen living in dormitories also are at increased risk of acquiring infection.
Meningococcal disease has a worldwide distribution. The incidence of disease varies from year to year because of the superimposition of 3- to 5-year epidemic cycles on the base of endemic disease activity. Since N. meningitidis was classified into serogroups in 1950, epidemic and endemic serogroups have been identified. Historically, group A strains were associated with worldwide epidemics until 1963, when a serogroup shift occurred, and epidemic group B disease was observed. These shifts have continued, to group C in 1967, and to groups A and B in 1976. Clusters of group C disease have been reported in schoolchildren after epidemic influenza. In sub-Saharan Africa, in an area called the “meningitis belt,” meningococcal infection is caused almost exclusively by group A; groups B and C cause the bulk of disease in other countries, although groups Y and W135 also occur.
The reasons for shifts in serogroup prevalence are unclear. One group of investigators described the clonal population structure of more than 400 strains of N. meningitidis group A that were isolated from 23 outbreaks. Most epidemics or outbreaks were characterized by a single or predominant clone. Similar clonal analyses have been carried out for serogroups B and C. A limited number of clones have been responsible for the epidemics since 1915. Hypothetically, epidemic outbreaks may begin only when changes in herd immunity coincide with the appropriate seasonal and climatic conditions that promote the carriage and transmission of one or more strains.
Sporadic cases usually are caused by serogroup B or C. Until recently, these infections were likely to be caused by group B strains in the 6- to 24-month age group and by group C strains in older children. In the United States, group Y disease, which often is associated with pneumonia and other focal manifestations of disease, has been observed with increasing frequency throughout the 1990s, and in one study, it accounted for 26% of cases observed from 1992 through 1996. Currently in the United States, group B predominates in children younger than 5 years of age, but more than two-third of cases in other age groups are attributable to groups C and Y.
Meningococcal disease occurs in a typical seasonal pattern worldwide. In the “meningitis belt,” disease recurs annually, in waves, with attack rates rising at the end of the dry season and declining after the rainy season begins. In the rest of the world, the attack rate peaks in winter and early spring months, a typical disease season in the Northern hemisphere running
from November through March. This seasonal pattern coincides with a concomitant rise in the incidence of viral upper respiratory tract infections, lending weight to the theory that viruses may act as cofactors in the development of invasive disease.
from November through March. This seasonal pattern coincides with a concomitant rise in the incidence of viral upper respiratory tract infections, lending weight to the theory that viruses may act as cofactors in the development of invasive disease.
Infections that develop within 24 hours of onset in the index case are designated as coprimary, whereas onset at least 24 hours after exposure to the index case is referred to as a secondary case. Sporadic cases are to be distinguished from a cluster of cases, in which two or more cases of the same serogroup occur more closely than expected in a population, and from an outbreak, in which increased transmission of infection in a population occurs.
PATHOGENESIS AND PATHOLOGY
Humans are the only natural host for meningococcal infection, and the oropharynx is its reservoir. Acquisition of nasopharyngeal infection by inhalation or direct contact results in transient, intermittent, or chronic carriage. The prevalence of asymptomatic carriage during nonepidemic periods ranges from 2% to 38%, and the median duration of carriage is 10 months. It is estimated that only 1% of carriers in Norway during the 1970s developed invasive disease. The carriage rate is increased in situations of overcrowding, in lower socioeconomic groups, and by passive and active smoking. In most hosts, infection of the upper respiratory tract elicits the formation of serum bactericidal antibody 7 to 10 days later. This immune response does not eliminate carriage, but it does protect the host from symptomatic infection. Susceptibility to invasive disease exists in the interval between acquisition of the organism in the nasopharynx and development of bactericidal antibody in the serum. Pili mediate the attachment of meningococci, and parasite-directed endocytosis promotes their entry into nonciliated cells of the nasopharyngeal mucosa. Dissemination occurs when the organism penetrates the nasopharyngeal mucosa of the nonimmune host and enters the bloodstream, where it replicates. From there, it may disseminate to the meninges, joints, myocardium, or elsewhere. Injury to the nasopharyngeal mucosa by preceding respiratory viral infection or smoking may promote invasiveness, but this hypothesis is contested.
The prevalence of passively or naturally acquired bactericidal antibody is inversely related to the incidence of invasive infection. Maternally derived antibodies probably provide some protection for most infants during the first few months of life. Passively acquired antibody concentrations reach a nadir between the ages of 6 and 24 months. Nasopharyngeal carriage of meningococci from serogroups with low pathogenicity may elicit cross-reactive antibodies that protect against invasiveness of pathogenic serogroups A, B, and C. Similarly, gastrointestinal colonization with bacteria containing antigens that cross-react with meningococci may contribute to the development of naturally acquired immunity.
Specific antibody and complement are important for immunity. Specific bactericidal IgG antibodies bind to meningococci and may activate the classic or alternative complement pathways. Bacterial killing can be mediated by serum, which requires the membrane attack complex, or by phagocytes. Patients who are deficient in specific antibody must rely more heavily on the integrity of complement-dependent bactericidal activity. Fatal or recurrent meningococcemia has been associated with congenital deficiencies of the alternative (properdin deficiency) or terminal complement pathways (components C5 through C9). These hosts must kill the organism by phagocytic rather than complement-mediated mechanisms. Defects in alternative and terminal complement pathways are easily assessed by the AP50 and CH50 assays, respectively. Partial compensation for this opsonic deficiency can be provided by eliciting specific antibodies through immunization. Acquired complement deficiency, as occurs with systemic lupus erythematosus (SLE), chronic liver disease, and nephrotic syndrome, also predispose to meningococcal infection. Some people develop serum IgA antibody (i.e., blocking antibody) that renders the bactericidal IgG or IgM antibody ineffective and results in disease susceptibility.
The predominant pathologic feature of fulminant meningococcemia is diffuse vascular damage and disseminated intravascular coagulation (DIC). In DIC, thrombin is persistently and recurrently elaborated, and fibrinogen, platelets, and coagulation factors such as anti-thrombin III and protein C (which may also have antiinflammatory effects) are consumed, with resulting haphazard activation and inhibition of fibrinolysis. Bleeding into any organ may occur. Histopathologically, the vascular changes consist of endothelial damage, vessel wall inflammation, necrosis, and thrombosis. These changes presumably are mediated by the effects of endotoxin, because the degree of septic shock correlates closely with endotoxin levels, and the effects are reproducible in animal models. A correlation between C3 activation products and the level of endotoxin supports the concept that complement activation contributes to multiple organ failure in overwhelming disease. Increased vascular permeability with leakage of plasma proteins, changes in vasomotor tone with maldistribution of intravascular volume, impaired myocardial function, and disordered cellular metabolism all interact to cause the circulatory collapse and myocardial dysfunction that is the major cause of death.
CLINICAL MANIFESTATIONS AND COMPLICATIONS
The clinical expression of meningococcal disease in children may be categorized as meningococcal bacteremia without sepsis, meningococcal sepsis without meningitis, meningitis, and other manifestations. The initial replication of meningococci in the bloodstream usually causes the nonspecific symptoms of fever, malaise, myalgia, and headache. Bacteremia without a focus may be considered as a possible diagnosis and, depending on the degree of toxicity, these patients may inadvertently be sent home, with or without antimicrobial therapy. When one group of 13 children in whom occult bacteremia had been diagnosed were reassessed, the bloodstream cleared without antimicrobial therapy in three patients, four developed meningitis, and the remainder were clinically improved with amoxicillin therapy. In another series of 37 children with unsuspected meningococcal infection when they first presented for medical attention, 17 subsequently developed meningitis, one hypotension, one respiratory failure, two pericardial effusion, and one died. Therefore, although some children with meningococcal bacteremia clear their bloodstream without antimicrobial therapy, the risks of developing meningitis (and/or other focal disease) or recurrent bacteremia with or without sepsis, warrant a full diagnostic evaluation including lumbar puncture and treatment with appropriate antimicrobials once meningococcal bacteremia is detected. Chemoprophylaxis also should be given to the contacts of these children.
Acute meningococcal bacteremia without meningitis begins with influenzalike symptoms (fever, upper respiratory signs, lethargy) that may last hours to days and are very nonspecific. Eventually, most affected children are septic. The majority of these children have cutaneous manifestations, which initially may take the form of a nonspecific maculopapular, morbilliform, or urticarial rash. Evolution to a petechial or purpuric
rash within hours or days is the rule. Purpura, usually most extensive on the buttocks and lower extremities, is a feature of fulminant disease (Fig. 165.1). In fulminant disease, the patient is toxic and ill-appearing and clinical deterioration is rapid, sometimes occurring within minutes. Hypotension, oliguria, DIC, myocardial dysfunction, and vascular collapse (often irreversible) lead to death in approximately 10% to 20% of the patients. When the course is less fulminant, and shock is responsive to therapy, the occasional fatal infection usually is due to the consequences of direct invasion of the myocardium, manifested by congestive failure, poor contractility, and pulmonary edema.
rash within hours or days is the rule. Purpura, usually most extensive on the buttocks and lower extremities, is a feature of fulminant disease (Fig. 165.1). In fulminant disease, the patient is toxic and ill-appearing and clinical deterioration is rapid, sometimes occurring within minutes. Hypotension, oliguria, DIC, myocardial dysfunction, and vascular collapse (often irreversible) lead to death in approximately 10% to 20% of the patients. When the course is less fulminant, and shock is responsive to therapy, the occasional fatal infection usually is due to the consequences of direct invasion of the myocardium, manifested by congestive failure, poor contractility, and pulmonary edema.
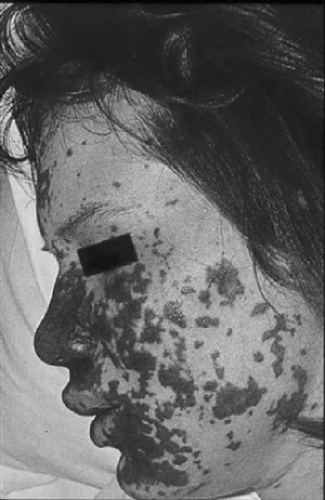 FIGURE 165.1. Purpura fulminans in a child with fulminant meningococcal sepsis. Purpura may evolve rapidly even in an initially non-toxic appearing child. |
Only one-third to one-half of the children with meningococcal meningitis have petechiae or purpura at the time of initial evaluation. Among the remainder, the clinical presentation often is that of bacterial meningitis, characterized (except in very young infants) by nuchal rigidity, altered level of consciousness, and signs or symptoms of increased intracranial pressure. Most children (95%) with meningococcal meningitis survive and have a better outcome than those with meningitis caused by Streptococcus pneumoniae. The most common cause of death is cerebral edema with herniation.
Children with meningitis or suspected meningococcal bacteremia have higher bacterial counts in their bloodstream than do those with unsuspected meningococcal bacteremia or other manifestations of infection. However, at the time of hematogenous dissemination, other sites may be seeded. The primary presentation reflects the particular focus in which a nidus for infection was established. Primary meningococcal pneumonia, periorbital cellulitis, pericarditis, peritonitis, cervical adenitis, endocarditis, purulent conjunctivitis, and endophthalmitis are rare, but they have been reported in children. Primary meningococcal infection of bone and joint (not be confused with immune complex–mediated disease) also has been described and is associated with benign outcome in most cases. Occasionally, manifestations of disease usually attributed to N. gonorrhoeae, such as vulvovaginitis or pelvic inflammatory disease, prove to be caused by N. meningitidis. The syndrome of chronic meningococcemia, in which persistent meningococcal bacteremia is associated with fever, skin lesions resembling gonococcemia, and arthritis, also is extremely rare in childhood.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







