CHAPTER 27
Management of the Cancer Patient With Spasticity
Adrienne R. Hill, Vishwa S. Raj, and Heather W. Walker
Tumors of the central nervous system (CNS) can cause spasticity due to insults to upper motor neurons within the brain parenchyma or from spinal cord injury (SCI). The tumors can be primary or metastatic and can lead to insufficient descending inhibition. Primary CNS tumors comprise different histologies and origins. The World Health Organization (WHO) has published a classification system for CNS tumors (1). This chapter highlights key aspects related to epidemiology, clinical presentation, types of CNS tumors, and other practical considerations related to spasticity treatment for cancer patients.
EPIDEMIOLOGY
The Central Brain Tumor Registry of the United States (CBTRUS) documents incidence, mortality, lifetime risk, survival rates, and prevalence of CNS tumors. The incidence rate of brain and CNS tumors is estimated to be 21.42 cases per 100,000, which total 343,175 tumors in the United States. Females have a higher incidence rate than males at 23.26 per 100,000 versus 19.42 per 100,000, respectively (2). The CDC predicts more than 68,000 new cases of CNS tumors in 2015 with 23,000 being primary malignant and 13,700 deaths expected (3). There is a 0.62% chance of acquiring a primary malignant brain/CNS lesion. Survival rates vary significantly according to type, patient age, and tumor behavior; however, it is estimated that 5-year survival of a patient diagnosed with primary malignant CNS tumor is 34.2% and 91.9% for nonmalignant CNS tumors (4). The prevalence rates for malignant and nonmalignant CNS tumors are 61.9 per 100,000 and 177.3 per 100,000, respectively (5).
The most common types of CNS tumors are gliomas and meningiomas. Gliomas account for 40% of all tumors and 78% of those with malignant tumors. Meningiomas comprise 30% of all tumors. Genetic conditions, exposure to ionizing radiation, and immunosuppression are the major risk factors for the development of CNS lesions (6).
CLINICAL PRESENTATION
Most CNS lesions present with either generalized or focal neurologic dysfunction. Patients may present with general complaints such as headache, seizure, bowel and bladder incontinence/retention, cognitive dysfunction or, more specifically, will present with focal weakness, sensory loss, gait ataxia, vision changes, or communication deficits. Symptoms are caused by direct tumor invasion, compression on surrounding tissue, and increased intracranial pressure (ICP).
Early in the disease process, muscle weakness may be subtle. Central tumors typically cause pyramidal deficits in which weakness predominates in the lower extremity flexors and upper extremity extensors. Supination weakness is often more pronounced than pronation, which accounts for pronator drift. Tumor-related pyramidal deficits are a result of descending motor tract disruption. Later in the disease process, motor weakness may become more apparent and spasticity may manifest as the inhibitory modulation of alpha motor neurons becomes more disrupted due to tumor burden.
Even though headache is a common complaint, isolated headaches are rarely the only presenting symptom (7). If the headache is accompanied by neurologic findings, change in headache pattern, or nausea and vomiting, then further workup is warranted. Tumor-related headaches usually are bifrontal but worse on the same side of the tumor, dull, constant and may be worse after increases in intrathoracic pressure. They tend to be worse at night possibly due to increases in PCO2 (8).
Seizure activity is the presenting symptom in up to 30% of patients diagnosed with a CNS tumor with increased risk correlated with tumor location and grade (9). Patients are at higher risk for seizure if the tumor is slowly progressive, low grade, and located in the gray matter. Seizures may be generalized or focal, may develop later in the disease course, and are typically repetitive and stereotyped. The majority of tumor-related seizures are focal in nature and vary widely from bizarre (déjà vu, sensation of fear, unusual smell) to common symptoms (isolated limb movements, posturing, etc) (6).
CNS lesions may cause nausea or vomiting due to medullary increased ICP. Tumor-related emesis is often associated with sudden change in body position. Increased ICP may also lead to decreased cerebral perfusion causing syncopal episodes particularly during Valsalva.
Patients with spinal cord tumors often present with nocturnal pain at the site of the lesion or difficulty with ambulation. Neurologic abnormalities such as dysesthesias and weakness occur distal to the site of the lesion as a result of impaired neural circuits. Symptoms may initially present unilaterally but may progress to bilateral involvement. Sphincter dysfunction may develop rendering the patient with bowel and bladder incontinence (10). Later in the disease course, patients may develop spasticity within selected muscles below the level of injury.
TYPES OF CNS TUMORS
Gliomas are lesions of the CNS with various degrees of progression and may ultimately lead to the development of spasticity. High-grade gliomas are considerably more common than low-grade gliomas. The WHO identifies tumors based on site of origin. Tumors arising from neuroepithelial tissue include astrocytic, oligodendroglial, oligoastrocytic, ependymal, choroid plexus, neuronal, pineal, and embryonal tumors. Tumors associated with cranial and paraspinal nerves include schwannomas, neurofibromas, perineuromas, and malignant peripheral nerve sheath tumors. Lesions that originate from the meninges are meningiomas, mesenchymal tumors, melanocytic lesions, and hemangioblastomas. Lymphomas and hematopoietic neoplasms such as plasmacytomas and granulocytic sarcomas as well as germ cell tumor may also result in altered neural function leading to increased tone in the extremities (1).
Primary spinal cord tumors represent 2% to 4% of all primary CNS lesions. They are classified based on location. Intramedullary tumors are located within the cord and are usually ependyomas or astrocytomas. Intradural-extramedullary tumors arise in the dura, outside the spinal cord, and are typically meningiomas and nerve sheath lesions. Extradural tumors arise outside of the cord, often within the vertebral bodies, and are metastatic in nature (10).
OTHER CONSIDERATIONS
Antispasticity agents may be used for cancer patients who develop increased tone as a result of their lesion; however, literature suggests other potential indications for their use in the oncologic population. For example, De Micheli et al reported on the use of BOTOX® for radiation-induced myokymia and proctitis (11). Similarly, radiation-induced trismus, facial pain, and masseter spasms were also improved in patients who had head and neck cancer (12). Stubblefield et al investigated the role of BOTOX as an adjunct treatment for radiation fibrosis syndrome (13).
As interest grows within the field of cancer rehabilitation, oncology providers are increasing referring their patients to rehabilitation physicians for management of spasticity, functional deficits, and other impairments.
Cancer rehabilitation is a medical care that should be integrated throughout the oncology care continuum and delivered by trained rehabilitation professionals who have it within their scope of practice to diagnose and treat patients’ physical, psychological, cognitive, and functional impairments in an effort to maintain or restore function, reduce symptom burden, maximize independence, and improve quality of life in this medically complex population (14).
Oftentimes, cancer treatments cause more undesirable functional and cognitive deficits than the cancer itself. Most cancer survivors need help in managing their symptoms years after their acute treatment has ended. The issues cancer patients struggle with are both physical and emotional. They may include spasticity, fatigue, pain, neuropathy, ataxia, reduced range of motion (ROM), difficulties with swallowing, talking, and eating, edema, emotional distress, anxiety, depression, as well as many others. These issues can profoundly affect quality of life, which can make the greater recovery very long and difficult.
Dr. Julie Silver, a physiatrist from Harvard Medical School and cancer survivor, cofounded Oncology Rehab Partners and has developed a framework for developing an institutional, multidisciplinary cancer rehabilitation program. The program is called STAR, which stands for Survivorship Training and Rehabilitation. The model consists of the following: patient screening before undergoing acute cancer treatments, early identification of impairments, prehabilitation if indicated, outcomes tracking, and treatment of functional deficits throughout the survivorship continuum. Prehabilitation is a process on the continuum of care that occurs between the time of cancer diagnosis and the beginning of acute treatment. It includes physical and psychological assessments that establish a baseline functional level, impairment identification, and interventions that promote physical and psychological health to reduce the incidence and/or severity of future impairments (Figures 27.1 and 27.2).
FIGURE 27.1 Survivorship care continuum.
Source: Reprinted from the STAR Program® and used with permission from McKesson Corporation and/or one of its subsidiaries. Copyright 2014. All rights reserved.
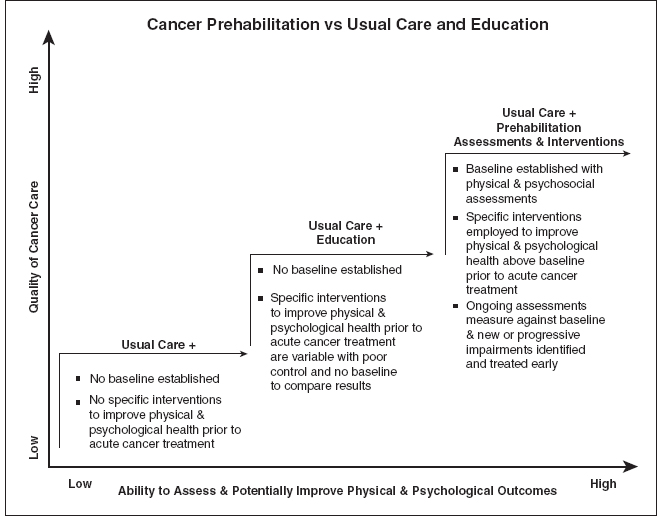
FIGURE 27.2 Cancer prehabilitation versus usual care and education.
Source: Reprinted from the STAR Program® and used with permission from McKesson Corporation and/or one of its subsidiaries. Copyright 2014. All rights reserved.
Impairment-driven cancer rehabilitation is a critical part of high-quality care. The American Cancer Society, the Institute of Medicine, and the Commission on Cancer mandate that cancer rehabilitation and survivorship care must be a distinct and well-developed part of cancer treatment. Cancer patients need specialized medical rehabilitation services designed specifically for their unique needs. The goal of a cancer rehabilitation program is to help cancer survivors have the best possible life every day during and after cancer. Cancer rehabilitation has been proven to be cost-effective and should be standard of care to reduce disability and maximize patients’ quality of life (15).
NONPHARMACOLOGICAL SPASTICITY MANAGEMENT
Although spasticity can effectively be managed in oncological patients with the use of modalities, oral medications, and interventional techniques, several variables may directly affect the safety and efficacy of management. Propagation of metastatic disease is a significant concern for any rehabilitation interventions. Several theories exist as to how cancer cells may spread to other organ systems. Although these cells may invade normal tissue by direct contact, metastases are generally spread through the vascular or lymphatic system (16). Cancer cells can enter the vascular system and then deposit to distant sites according to blood flow. Similarly, these cells can migrate after invasion of the lymphatic system (17). When using modalities, such as heat, ultrasound, and laser treatments, there is a theoretical risk that increased energy applied to areas of tumor growth may cause vasodilation and increased blood flow, which in turn may lead to higher risk of tumor seeding and dissemination. This concern must be weighed against the benefits of tone reduction in spasticity.
In addition, when using bracing techniques to help with spasticity management, considerations should be made for bone stability and the risk of pathological fracture. The Harrington and Mirels models have traditionally been used to determine orthopedic stability for patients (18,19). However, no clear guidelines exist to guide bracing needs for spasticity in coordination with osteopenia and risk of pathological fracture for cancer patients. Given the physical forces involved with bracing for tone management, a thorough evaluation should be performed if there is any concern of bony insufficiency. Orthopedic evaluation may also be necessary to clarify the weight-bearing and ROM status, as well as potential surgical options to improve stability before more aggressive rehabilitation protocols are initiated.
Chemotherapy-induced peripheral neuropathy is a common cause for alterations in sensation for the cancer patient. Several medications, such as Vinca alkaloids (20), platinum-based compounds (21), and microtubule-stabilizing agents, can cause nerve damage (22). Sensory nerves are typically affected first because of decreased myelination and smaller size (23). Considerations should be made if using bracing for spasticity, as individuals may not perceive abnormal distributions of pressure, which could then lead to skin breakdown. Clinicians should be focused on both positioning and posture when prescribing orthotic devices (24).
PHARMACOLOGICAL TREATMENT
Several medications are approved for the treatment of clinical spasticity resulting from upper motor neuron disorders (eg, SCI, stroke, cerebral palsy, or multiple sclerosis [MS]), though none specifically for cancer-related spasticity. The more commonly used agents, which are Food and Drug Administration (FDA) approved for the treatment of spasticity, include baclofen, diazepam, dantrolene sodium, and tizanidine (25), but other options are discussed as well.
Individuals living with cancer often suffer from acquired cognitive dysfunction secondary to chemotherapeutic treatment. It can be a consequence of the cancer, its treatment, or both (26). Several oral medications used in the treatment of spasticity, however, can contribute to worsening cognitive functioning. Adverse effects of baclofen, a gamma-aminobutyric acid (GABA)-B receptor agonist, include sedation and somnolence (27). Benzodiazepines, such as diazepam, act as GABA-A antagonist and can also have CNS depressant effects as it relates to cognition and psychomotor performance. Furthermore, alpha-2 blockers such as tizanidine have been shown to cause drowsiness. The propensity for tizanidine to decrease blood pressure should also be considered when evaluating cognitive decline. As all of these medications are cleared through the renal or hepatic systems, tumor involvement in and impairment of the respective system should be factored into dosing protocols (28). Each of these medications and the potential side effects are discussed in more detail in the following.
When initiating treatment for spasticity in individuals with a primary diagnosis of cancer, it is appropriate to take a graduated approach, and employ noninvasive, therapeutic techniques, such as stretching, and use of modalities discussed previously, before initiation of pharmaceutical agents. However, with the involvement of the CNS due to primary or metastatic disease process, individuals with cancer may present with focal or generalized spasticity that requires medication administration for appropriate management. It is useful to be familiar with the agents that are most commonly used to treat spasticity; additionally an understanding of the mechanism of action, therapeutic dosing, and potential side effects can guide treatment in this patient population.
Baclofen (Lioresal®)
Baclofen is an antispasticity agent that is commonly used in the treatment of spasticity. Baclofen decreases monosynaptic and polysynaptic reflexes by binding presynaptically to the GABA-B receptors in the brain and spinal cord. Binding of baclofen to the GABA-B receptors decreases calcium influx into the presynaptic terminal; this in turn decreases the release of excitatory neurotransmitters. The decreased release of excitatory neurotransmitters by the interneurons and afferent fibers is responsible for the diminution of reflex activity (29). Binding of baclofen to GABA-B receptors may also decrease gamma motor neuron activity, which may decrease muscle spindle activity (25).
Baclofen is a relatively short-acting agent, with a mean half-life of approximately 3 hours (range, 2–6 hours). Due to the short half-life, it is often necessary to provide dosing three to four times daily. Baclofen is excreted primarily by the kidney; however, 15% is metabolized by the liver; therefore, caution must be exerted when using baclofen in patients with renal insufficiency, and liver function tests should be monitored (25). Additionally, in cancer patients taking chemotherapeutic agents processed through the liver, or patients with known primary or metastatic liver involvement, extra caution should be taken.
Baclofen is typically initiated at a dose of 5 mg two to three times daily and gradually titrated up to an appropriate dose based on patient response and side effects, with a maximum daily-recommended dose of 80 mg per day; however, higher doses have been used in some patient populations. Common side effects noted with the use of baclofen include sedation and mental confusion, ataxia, hypotonia, muscle weakness, and constipation. More significant effects have been reports with overdose, including respiratory failure, seizures, coma, and death. Baclofen should not be abruptly discontinued, as this can result in seizures, hallucinations, and potentially death.
Benzodiazepines
Diazepam (Valium®
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






