Degenerative disk disease is a strong etiologic risk factor of chronic low back pain (LBP). A multidisciplinary approach to treatment is often warranted. Patient education, medication, and cognitive behavioral therapies are essential in the treatment of chronic LBP sufferers. Surgical intervention with a rehabilitation regime is sometimes advocated. Prognostic factors related to the outcome of different treatments include maladaptive pain coping and genetics. The identification of pain genes may assist in determining individuals susceptible to pain and in patient selection for appropriate therapy. Biologic therapies show promise, but clinical trials are needed before advocating their use in humans.
Low back pain (LBP) affects every population and is one of the world’s foremost debilitating conditions. Such pain may lead to diminished function and quality of life, psychological distress, and loss of wages. LBP is one of the most common conditions motivating individuals to seek medical care and often results in prolonged therapeutic interventions. Therefore, LBP is a global burden associated with severe socioeconomic and health care consequences.
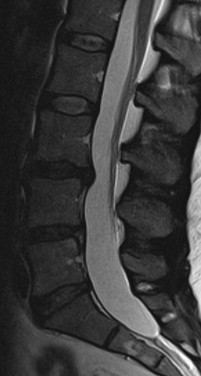
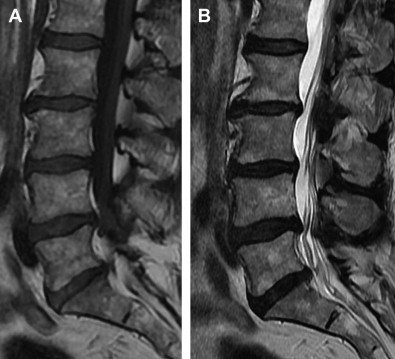
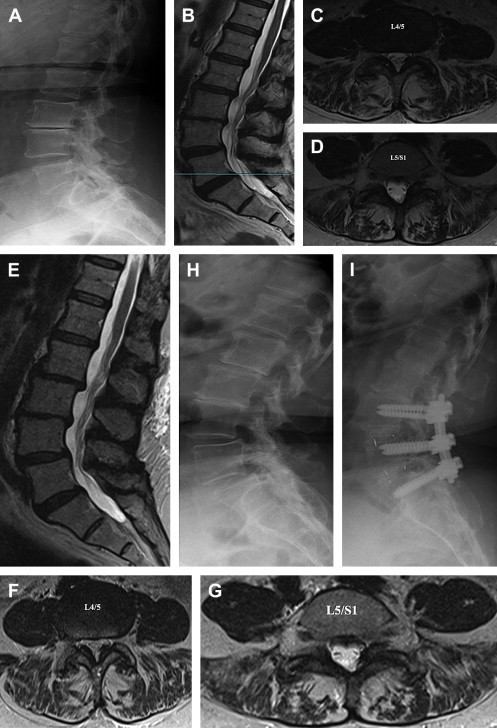
LBP can be divided into several groups based on cause: 80% to 90% mechanical (eg, degenerative disk or joint disease, vertebral fracture, deformity); 5% to 15% neurogenic (eg, herniated disk, spinal stenosis), 1% to 2% nonmechanical conditions (eg, neoplastic disease, infection, inflammatory), 1% to 2% referred visceral pain (eg, gastrointestinal disease, renal disease, abdominal aortic aneurysm), and 2% to 4% other (eg, fibromyalgia, somatoform disorder, malingering). Typically, patients with LBP complain of local pain aggravated by mechanical loading, usually at worst when being upright, and they have no or minimal symptoms at rest. It is generally agreed that intervertebral disks are a major tissue source in chronic LBP. Typically, chronic LBP has been defined as pain occurring for 3 months or more, frequently recurring, or lasting beyond the normal healing period for a low back injury. If, in case of prolonged LBP, magnetic resonance imaging (MRI) is obtained and a common finding is disk degeneration at the 2 or 3 lowest lumbar levels ( Figs. 1–3 ).
According to international clinical guidelines, treatment of acute LBP (ie, <3 months) is straightforward in the absence of red flags ( Table 1 ) or sciatica symptoms. Often, pain medication is provided and the patient is advised to stay active. However, in the context of chronic LBP, there are several treatment options, but no clear answer exists as to how the physician should plan the treatment process. This article reviews treatment options for the management of chronic LBP and assesses the evidence on their effectiveness, with particular emphasis on degenerative disk disease.
| Condition | History | Physical Examination |
|---|---|---|
| Fracture | Major trauma Minor trauma (older patient) | Kyphosis |
| Tumor | Age <15 or >50 y Known cancer Unexplained weight loss Night pain | — |
| Infection | Recent fever or chills Recent bacterial infection(urinary tract infection) Intravenous drug use Immune suppression Unrelenting pain | Fever |
| Cauda equina syndrome | Saddle numbness Urinary retention, incontinence Severe (progressive) lowerextremity neurologic deficit | Weak anal sphincter Perianal sensory loss Flaccid motor weakness Hyporeflexia |
The role of disk degeneration in chronic LBP
MRI is not recommended early in the disease course unless red flags or signs of nerve root entrapment are present. The reason is that MRI in acute LBP increases medical costs without giving additional information influencing clinical decision making. Furthermore, MRI in the current form is not useful in diagnosing discogenic pain when compared with discography. However, discography per se has been found to enhance progression of disk degeneration, and therefore recently published guidelines were not in favor for discography. According to Ohtori and colleagues, injection of a small amount of bupivacaine into the painful disk may be a better test for discogenic LBP than discography. However, this procedure is also invasive and may accelerate disk degeneration. Therefore, in most cases of chronic LBP the true tissue origin has remained unknown. In most randomized trials focused on patients with chronic LBP, the tissue source of pain has not been speculated.
According to a systematic review by Hancock and colleagues, MRI findings, such as endplate changes and presence of disk degeneration, were found to increase the likelihood of the discogenic origin from discography. Several recent studies support the concept that disk degeneration is associated with low back symptoms. All these studies indicate that a higher degree of lumbar disk degeneration is related to a higher likelihood of symptoms, and moreover the presence of moderate disk degeneration or degenerative changes at multiple levels increases the likelihood of pain. According to Samartzis and colleagues, the global severity of disk degeneration increases the likelihood of LBP, with a potential dose-response exposure of degenerative changes implicated in the association.
The role of disk degeneration in the development of chronic LBP has received considerable attention; nonetheless, few large-scale studies have addressed the relationship. According to studies by Kjaer and colleagues, Visuri and colleagues, and Paajanen and colleagues, disk degeneration on MRI is significantly associated with chronic LBP, whereas Savage and colleagues contend otherwise. More recently, a systematic review by Chou and colleagues assessing degenerative spine findings on MRI in relation to chronic LBP, noted a significant association between the presence of disk degeneration and back pain. However, because of clinical heterogeneity between studies, the investigators hesitated in making any robust conclusions of a direct association or causal pathway between disk changes and LBP. Nonetheless, a recent study by DePalma and colleagues using numerous diagnostic injections concluded that intervertebral disk degeneration is the most common tissue source of chronic LBP. The likelihood of the intervertebral disk implicated in chronic LBP was highest in young and middle-aged individuals, whereas the probability of pain related to facet or sacroiliac joints was highest in older individuals. In addition, new imaging modalities, such as T1-ρ, T2-relaxation mapping, and chemical exchange saturation transfer, are being developed that are more sensitive to disk changes and could further elaborate more quantitatively on the disk degeneration phenotype as well as possess the potential to image pain (see article by Majumdar and colleagues elsewhere in this issue).
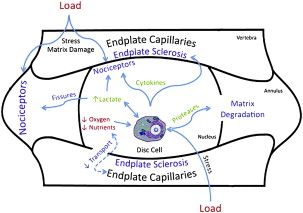
In this article, presumed discogenic origin of chronic LBP is referred to as degenerative disk disease. The pathophysiologic mechanism leading to the development of pain in the disk is described elsewhere in this focus issue (see articles by Chan and colleagues, Grunhagen and colleagues, Inoue and Espinoza Orias, and Bae and Masuda). In general, mechanical and chemical mediators brought on by the degenerative process irritate sensory nerve endings (nociceptive fibers) located in the annulus fibrosus, which contribute to pain ( Fig. 4 ). As the degenerative process progresses, this situation may further affect the kinematics and load transmission throughout the motion segment, thereby stimulating nociceptive fibers in the facet joints as well.
The role of central sensitization in chronic LBP
Nociceptive stimuli from peripheral tissue, such as in the intervertebral disk, are transmitted mainly via the spinothalamic tract to the cerebral cortex. In case of persistent injury, C fibers fire repetitively to the dorsal horn, which may lead to central sensitization. Central sensitization is characterized by altered pain sensibility both peripherally and centrally. Even although intervertebral disks are the original pain generators in degenerative disk disease, central sensitization may obscure a peripheral nociceptive tissue source in chronic LBP. The central areas activated by pain include almost constantly secondary somatosensory cortex, insular regions and anterior cingulate cortex, and with slightly less consistency contralateral thalamus and primary somatosensory cortex. There is reasonable evidence that chronic LBP is associated with abnormal brain anatomy and function, especially in the dorsolateral prefrontal cortex, thalamus, brainstem, primary somatosensory cortex, and posterior parietal cortex. According to a study by Ruscheweyh and colleagues that assessed structural MRI of the brain and pain status in 205 German subjects, regional brain matter reduction (mainly in cingulate, prefrontal, and motor/premotor regions) was present in chronic LBP sufferers with symptoms greater than 12 months. However, a recent Canadian study by Seminowicz and colleagues indicated that brain abnormalities in chronic pain may be reversible. These investigators reported that successful treatment of patients with chronic LBP either with spine surgery (n = 8) or with a facet joint injection (n = 6) resulted in restoration of both structure and function of the left dorsolateral prefrontal cortex, which correlated with reduction of both pain and disability.
The role of central sensitization in chronic LBP
Nociceptive stimuli from peripheral tissue, such as in the intervertebral disk, are transmitted mainly via the spinothalamic tract to the cerebral cortex. In case of persistent injury, C fibers fire repetitively to the dorsal horn, which may lead to central sensitization. Central sensitization is characterized by altered pain sensibility both peripherally and centrally. Even although intervertebral disks are the original pain generators in degenerative disk disease, central sensitization may obscure a peripheral nociceptive tissue source in chronic LBP. The central areas activated by pain include almost constantly secondary somatosensory cortex, insular regions and anterior cingulate cortex, and with slightly less consistency contralateral thalamus and primary somatosensory cortex. There is reasonable evidence that chronic LBP is associated with abnormal brain anatomy and function, especially in the dorsolateral prefrontal cortex, thalamus, brainstem, primary somatosensory cortex, and posterior parietal cortex. According to a study by Ruscheweyh and colleagues that assessed structural MRI of the brain and pain status in 205 German subjects, regional brain matter reduction (mainly in cingulate, prefrontal, and motor/premotor regions) was present in chronic LBP sufferers with symptoms greater than 12 months. However, a recent Canadian study by Seminowicz and colleagues indicated that brain abnormalities in chronic pain may be reversible. These investigators reported that successful treatment of patients with chronic LBP either with spine surgery (n = 8) or with a facet joint injection (n = 6) resulted in restoration of both structure and function of the left dorsolateral prefrontal cortex, which correlated with reduction of both pain and disability.
Treatment of chronic LBP
Existing clinical guidelines list several treatment options for chronic LBP, which include pain medication, exercises, behavioral therapy, multidisciplinary rehabilitation, and surgery ( Box 1 ). Patient information is not reviewed in detail here. Yet, patient advice is an integral part of care at all stages. Such advice should preferably be given early in the disease course, because 2.5-hour sessions of individual oral education were found to be more effective than no intervention in return to work in subacute LBP, whereas in chronic LBP education was less effective on back-related function than more intensive interventions. Advice includes information on the benign nature of nonspecific LBP and encourages the patient to be physically active and continue with normal activities as possible.
Previous episode of back pain a,c
Poor job satisfaction or low pay a,c
Inadequate coping skills c
Fear-avoidance behavior a,c,d
Manual labor or physically stressful job a,c
Obesity a,c
Somatization a,c
Smoking a,c
Low baseline activity levels a,c
Ongoing litigation c
Older age a,c
Low educational level c
Higher pain intensity or disability c
Neurologic symptoms c
Anxiety a,c
Depressed mood c
Emotional distress a,c
Pain genes b
Association does not imply causality. Evidence is mixed for some factors, including smoking, obesity, and low educational level.
a Associated with development of LBP in some studies.
b Associated with pain severity after surgery. Limited studies exist.
c Associated with persistence of LBP in some studies.
d The avoidance of physical activities that stems from patients’ fears that their pain will worsen.
Some new promising biologic treatment alternatives have been introduced recently. They include stem cell regeneration, gene therapy, tissue engineering, and molecular therapy. All these treatments are reviewed elsewhere in this issue (see articles by Sakai, Woods and colleagues, Leung and colleagues, and Bae and Masuda). This article pays special attention to the following treatment domains: pain medication, exercise therapy, behavioral therapy, multidisciplinary rehabilitation, injection therapy, and surgery.
Initial Clinical Assessment
In the initial assessment, primary health care services, which include occupational health care in those countries where it is available, are of importance. A thorough clinical examination is paramount because it serves both the needs of diagnostics, and is also a part of evidence-based pain treatment. It is generally recommended that every patient with LBP should be examined carefully, with follow-up visits in case of prolonged or recurrent pain ( Table 2 ). Degree of baseline disability (rather than pain intensity) is an important prognostic factor for recovery of LBP. Functional impairment can be best evaluated with thorough clinical examination. In addition, patient-reported disability indices, such as the Oswestry Disability Index and the Roland-Morris Questionnaire, are helpful and widely used in the clinical assessment. A further tool in the initial assessment of patients with LBP is pain drawing, which is a simple and inexpensive diagnostic measure to characterize an abnormal psychological profile.
| Nonsurgical Treatment Alternatives | |
|---|---|
| Treatment | Subclassification |
| Education | – |
| Medication | Analgesics Nonnarcotic Narcotic Topical NSAIDs Muscle relaxants Corticosteroids Antidepressants |
| Cognitive behavioral therapy | Operant Cognitive Respondent |
| Multidisciplinary rehabilitation | – |
| Immobilization and supports | – |
| Exercise therapy | – |
| Massage therapy/physical therapy | – |
| Acupuncture/dry needling | – |
| Manipulation | – |
| Traction | – |
| Injections | Epidural Facet Trigger point Sacroiliac Intradiscal Prolotherapy |
| Orthoses | Braces Corsets Unloading corset |
| Transcutaneous electrical nerve stimulation | – |
| Acupuncture | – |
Pain Medication
The clinical guidelines recommend paracetamol as the first medication choice and nonsteroidal antiinflammatory drugs (NSAIDs) or weak opioids, or both, if paracetamol alone does not provide sufficient pain relief. NSAIDs are effective for short-term symptom relief in patients with chronic LBP without sciatica, but the effect sizes are small and the various types of NSAID are equally effective. In addition, the clinician should evaluate the risk of side-effects in each individual case and take into account the patient’s preference as well. In case of persistent pain, strong opioids can be used for short-term management. Overall, the benefits of opioids for long-term management of chronic LBP remain questionable. In addition, early use of opioids for LBP patients increases risk of work disability and leads to overall poor outcomes. Tricyclic antidepressants may be offered if other drugs are insufficient in pain relief ; however, there is no evidence on their efficacy in chronic LBP.
Exercise Therapy
Exercise therapy is the key element in the treatment of chronic LBP. Exercise therapy is effective at decreasing pain and improving function. However, exercise therapy was noted to have only a modest effect size and most statistically significant trial results on the efficacy of exercise in chronic LBP were not of clinical importance.
Selecting the type of exercise therapy for optimum effectiveness for chronic LBP is of importance. According to a meta-regression analysis by Hayden and colleagues, exercise therapy should consist of individually designed programs, include stretching or strengthening, and should be delivered with supervision. In addition, high-dose exercise programs fared better than low-dose exercise programs. In general, no specific exercise type was superior to other types. However, patient populations in the trials have been heterogeneous, whereas treatment interventions based on validated classification systems may result in larger effect sizes for the given treatments. Moreover, exercise therapy may not be tolerated by all patients with degenerative disk disease (at least at advanced degenerative disease). Patients with type I and mixed types I/II Modic changes do not respond well to exercise therapy.
The role of exercise therapy is supported by a review on the effectiveness of exercises for prevention of recurrences of LBP. The review found moderate-quality evidence that posttreatment exercise programs can prevent recurrences of LBP. Additional exercise programs after formal treatment of LBP has been completed are beneficial. However, evidence on treatment interventions, defined as treatment of a current episode of LBP with the aim to prevent new episodes of pain, was conflicting.
Behavioral Therapy
The main behavioral treatment approaches in chronic LBP are operant, cognitive, or respondent therapies (see Table 3 ). There is moderate evidence that operant therapy is more effective than waiting list, and that behavioral therapy in general is more effective than usual care in short-term pain relief in chronic LBP. The strength of evidence on the efficacy of behavioral therapy was found to be mostly of low quality.
| Behavioral Treatment Approaches | |
|---|---|
| Type | Definition |
| Operant | Removes positive reinforcement of pain behaviors and promotes healthy behaviors |
| Cognitive | Identifies and modifies harmful cognitions, such as maladaptive thoughts, feelings, and beliefs about LBP, using cognitive restructuring techniques (eg, imagery and attention diversion) |
| Respondent | Modifies the physiologic responses to pain through reduction of muscular tension using different relaxation techniques |
Two high-quality trials, published after the systematic reviews, suggest that cognitive therapy is an essential part in the treatment of chronic LBP. In a Danish pragmatic trial, a cognitive, educational intervention for chronic LBP resulted in at least as good outcomes as exercise therapy despite fewer treatment sessions. Moreover, they used a classification system in which the delivery of specific exercise therapy was based on assessment findings. According to a British multicenter study by Lamb and colleagues, cognitive behavioral therapy was found to significantly improve back-specific function compared with the usual care in subacute or chronic LBP. Furthermore, the effect was sustained over the 1-year follow-up period. In the intervention group, participants attended a program that targeted behaviors and beliefs about physical activity and avoidance of activity and consisted of individual assessment (up to 1.5 hours in duration) and 6 sessions of group therapy (1.5 hours per session).
Multidisciplinary Rehabilitation
Multidisciplinary rehabilitation has been defined to include multidisciplinary biopsychosocial rehabilitation coupled with a minimum of 1 physical dimension (ie, psychological or social or occupational). There is strong evidence that intensive multidisciplinary biopsychosocial rehabilitation with functional restoration improves function, and there is moderate evidence that multidisciplinary rehabilitation with functional restoration reduces LBP when compared with less intensive treatments. More recently, moderate evidence of multidisciplinary rehabilitation compared with other kinds of active treatment on pain intensity in the short-term was found ; however, no effect on pain intensity in the long-term was observed.
The optimal content of multidisciplinary rehabilitation remains to be defined. Behavioral therapy is widely considered to be an essential part of multidisciplinary rehabilitation, but the addition of behavioral therapy to inpatient rehabilitation did not seem to increase the effect of inpatient rehabilitation alone. Similarly, the addition of cognitive behavioral therapy did not increase the efficacy of physical conditioning. Multidisciplinary rehabilitation can be performed as outpatient rehabilitation as well. Based on a study by Lambeek and colleagues addressing a Dutch population, multidisciplinary outpatient work-related intervention was effective in return to work.
Based on systematic reviews by Guzman and colleagues and Ravenek and colleagues, there is contradictory evidence regarding vocational outcomes after multidisciplinary rehabilitation. In addition to multidisciplinary rehabilitation, physical conditioning programs, sometimes referred to as work conditioning, work hardening, or functional restoration/exercise programs, have a small effect on sickness absence at long-term follow-up in workers with chronic LBP. Return to work should be a feasible and realistic outcome of multidisciplinary rehabilitation according to Buijs and colleagues, who used a multidisciplinary outpatient care program, including workplace intervention and graded activity aiming at function restoration (instead of pain elimination) and return to work. Their program was well accepted by patients. Patient expectations were low at the start but the program was successful in changing patients’ goal setting from pain-oriented toward function restoration and return to work. In support of the positive effect of multidisciplinary rehabilitation on vocational outcomes, a high-quality Dutch trial by Lambeek and colleagues addressing patients who were on sick leave because of chronic LBP reported significantly less median duration of days until sustainable return to work in the so-called integrated care group (88 days) compared with to the usual care group (208 days). The integrated care intervention included a workplace intervention based on participatory ergonomics and a graded activity program based on cognitive behavioral principles, whereas the multidisciplinary team consisted only of a clinical occupational physician, a medical specialist, an occupational therapist, and a physiotherapist.
Injection Therapy
There is insufficient evidence to support epidural and facet joint injections, or local trigger point injections, in subacute and chronic LBP. A recent practice guideline by Chou and colleagues recommended against facet joint steroid injections, prolotherapy, and intradiscal steroid injections in nonradicular LBP, and strongly recommends against provocative discography. Epidural or transforaminal steroid injection is recommended in patients with persistent radiculopathy caused by a herniated lumbar disk because there is evidence for moderate short-term benefits. Furthermore, the benefits of botulinum and epidural steroid injection, intradiscal electrothermal therapy, therapeutic medial branch block, radiofrequency denervation, intrathecal therapy with opioids or other medications, and sacroiliac joint steroid injection are questionable in nonradicular LBP.
It could be argued that intradiscal injections with other more potent antiinflammatory drugs than steroids could be beneficial in nonradicular LBP. Tumor necrosis factor α (TNF-α) antagonists are eagerly evaluated in the treatment of sciatica. However, the current evidence does not support their use in degenerative disk disease. Fibrin injection in the experimentally damaged disks resulted in reduced TNF-α synthesis. No in vivo human studies have been performed. In addition, various growth factors and stem cell therapies that entail direct injection into the disk for repair/regeneration have been studied, largely in animal models and in disks with mild to moderate degeneration (see articles elsewhere in this issue by Sakai, Woods and colleagues, Leung and colleagues, and Bae and Masuda). Although their effectiveness for pain management in symptomatic degenerated disks has not been fully addressed, such disk therapies could serve as a viable option in the future and warrant further investigation.
Peng and colleagues reported their findings based on their randomized controlled trial (RCT) assessing the efficacy of methylene blue intradiscal injection (n = 36) compared with a placebo group (n = 36) in 72 patients with chronic discogenic LBP lasting longer than 6 months. These investigators noted at 24-month postinjection follow-up that intradiscal injection of methylene blue significantly reduced mean pain and Oswestry Disability Index scores by 41 and 35, respectively, among patients with chronic discogenic pain compared with 1% and 2%, respectively, in the placebo group. The investigators concluded that methylene blue acts to denervate the nociceptive fibers found in annular fissures. However, the study has not been replicated. Thus, the benefit of methylene blue injection remains speculative. Alternatively, although Peng and colleagues reported their procedure to be safe, an animal study performed by O’Neill and colleagues noted that methylene blue if leaked out of the disk and into the epidural space may prove extremely neurotoxic, resulting in paralysis in their animal models. O’Neill and colleagues have advocated that until the exact mechanism of toxicity and dose response of the relation are determined, the use of methylene blue to address symptomatic degenerative disk disease should be avoided or at least used in the setting of an intact annulus fibrosus that may diminish the risk of leakage of the injected agent.
Surgery
Surgery is an option for patients with degenerative disk disease nonresponsive to conservative treatment (see Figs. 1–3 ). Although controversial, in the carefully selected patient, lumbar spinal fusion may be regarded as the gold standard of surgical treatment of degenerative disk disease. Spinal fusions are a relatively common spine procedure that continues to grow in popularity. According to Rajaee and colleagues, the rate of spinal fusion has increased 2.4-fold from 1998 to 2008 in the United States.
Because pain relief has been achieved in other arthritic joints of the body through the elimination of painful motion, it has been assumed that analogous relief can be achieved through a successful spinal fusion. In a multicenter randomized trial, Fritzell and colleagues compared 3 common surgical techniques (posterior only, anterior only, and combined anterior posterior approaches) used to achieve a lumbar fusion. In this study, all fusion techniques were found to reduce pain and improve function, but there was no difference among the techniques used to achieve fusion. The investigators concluded that immobilization of the motion segment appeared to be the important component, whereas the surgical technique used appeared to be less important. Similarly, the use of instrumentation also remains unclear. Meta-analysis and randomized, prospective studies have suggested that although fusion rates are increased with pedicle screw fixation, an improvement in clinical outcomes may not be noted. Conversely, several have advocated that specific appropriateness criteria may improve surgical outcomes in patients with LBP. Nonetheless, according to a systematic review by Chou and colleagues, fusion is no more effective than intensive conservative rehabilitation for degenerative disk disease. Furthermore, fusion was associated with small to moderate benefits compared with standard (nonintensive) conservative therapy. Moreover, based on the Medical Research Council Spine Stabilization RCT assessing patients with chronic LBP at a minimum of 1-year duration who were randomized to undergo lumbar fusion or an intensive rehabilitation program based on cognitive behavioral principles, no difference in disability and functional outcome was noted in both treatment groups.
The efficacy of total disk replacement (TDR) has been scrutinized throughout the years (see article by Mayer and Siepe elsewhere in this issue). Based on a systematic review by van den Eerenbeemt and colleagues, it was concluded that studies assessing the efficacy of TDR lacked proper control groups and were generally of low quality. The results indicate that TDR is at best only of similar efficacy to lumbar fusion. In clinical practice, TDR is used mostly for single-level disk disease and not for multilevel disease. Nevertheless, the investigators concluded that the existing evidence, specifically regarding long-term effectiveness or safety, is considered insufficient to justify the widespread use of TDR for single-level degenerative disease. Furthermore, the correlation between radiographic evidence of motion preservation and clinical improvement in pain intensity has not been completely supported. In a recent prospective study addressing TDR by Blondel and colleagues, superior clinical outcomes based on Oswestry Disability Index and pain scales were observed in individuals with Modic type I endplate changes on MRI compared with Modic type II or no Modic changes. The findings from this study have stressed the importance of proper patient selection in individuals undergoing TDR to maximize surgical outcomes.
In general, rehabilitation is needed after disk surgery. Exercise programs starting 4 to 6 weeks after surgery seem to lead to a faster decrease in pain and disability than no treatment. Moreover, high-intensity exercise programs seem to lead to a faster decrease in pain and disability than low-intensity programs. No systematic reviews are available to assess the efficacy of rehabilitation regime after lumbar fusion surgery, but 1 recent RCT by Abbott and colleagues found a beneficial effect for rehabilitation after lumbar fusion surgery. However, the investigators concluded that in addition to neuromuscular exercises, rehabilitation should also address maladaptive pain coping.
Newer surgical techniques focusing on the use of dynamic stabilization have been described for the management of degenerative disk disease. Several systems currently exist and can be subdivided into 4 groups: (1) dynamic interspinous spacers, (2) static interspinous spacers, (3) pedicle screw/rod-based posterior dynamic stabilizing system, and (4) total facet replacement systems. Theoretically, they all attempt to address the degenerative segment through either direct distraction forces to unload the disk, or to shield the disk and facet joints from motion, or reduce facet contact and pressure. High-quality RCTs and systematic reviews on the role and outcomes of these systems on the management of degenerative disk disease are absent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







