Fig. 6.1
Reticular bone bruising, distant from the articular surface. Top arrow indicates bruising of the distal tibia and the lower red arrow indicates bruising of the talus
Mechanical malalignment overloads either the medial or lateral boarders of the talus depending if there is tibial or hindfoot varus or valgus [8]. This may affect healing of a defect or retard surgical repair. OCLs in the knee and the ankle have been shown to regress with high tibial realignment osteotomy or calcaneal osteotomies [9]. Similarly, ankle fracture malunion increases the contact pressures [10]. Ankle instability repetitively increases focal load and OLT propagation [11]. This should be addressed in the treatment plan when applicable (Fig. 6.2).
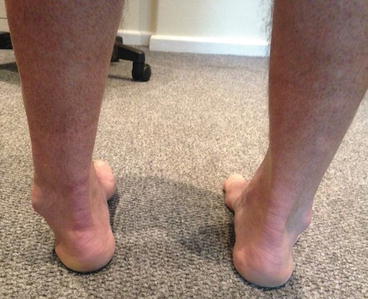

Fig. 6.2
Varus malalignment
Some lesions enlarge and become massive (>3 cm2) (Fig. 6.3) [12]. These are either central and contained or peripheral and uncontained. Sometimes the subtalar joint is involved. Although OLTs are slowly progressive or static and do not commonly lead to osteoarthritis, when large, point loading may lead to kissing lesions, involvement of the tibial plafond and secondary arthritis [13].
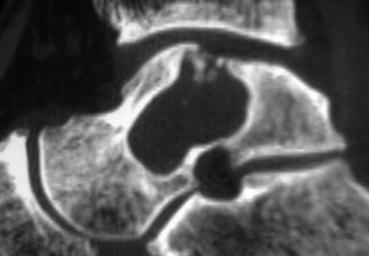

Fig. 6.3
Massive (>3 mm) cystic lesion
6.3 Clinical Presentation
Large uncontained lesions are usually painful as the structure of the talus is threatened. Point loading and loose bodies cause a synovitis, and the mechanics of the joint are altered. Some smaller cystic lesions are incidental findings, remain stable and quiescent and do not require treatment, but should be monitored with serial radiology [14]. Pain from symptomatic OCLs is typically deep, activity related with little swelling unless there is a loose body or cartilage flap. Some describe clicking or other mechanical symptoms in the joint. The majority of patients report a previous ankle sprain or multiple episodes of instability.
6.4 Investigation
Large cystic OTLs are usually visible on plain radiographs, but smaller central lesions can be difficult to see or are not visible (Fig. 6.4). MRI best illustrates the cartilage and surrounding bone oedema, but a CT scan is essential to understand the anatomy of the lesion, see if the cyst is contained and determine the involvement of the ankle and the subtalar joints. A plantarflexion CT is very helpful to plan the surgical approach and the need for an osteotomy in the case of an open procedure [15].
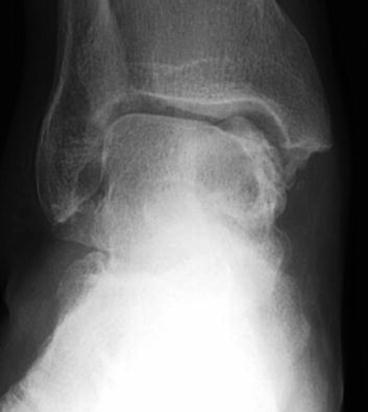

Fig. 6.4
Large cystic lesion on plain radiograph
6.5 Nonoperative Treatment
Rest, restricted sporting activities and casting aim to unload the cartilage and the subchondral bone allowing healing. The success rate, based on a few studies from two decades ago, is low, ranging from 29 to 58 % [16]. In the paediatric population, healing was thought to be superior [17], but a recent study showed that 92 % of initially conservatively treated children required surgery eventually [18]. If the cystic component is large and there is significant reactive bone oedema, nonsurgical treatment is less likely to be successful [3]. Rarely, a large cyst is identified in a relatively asymptomatic individual. This poses a treatment dilemma as an enlarging cyst can threaten the talar structure and be very difficult to manage once they do become symptomatic or the subtalar joint is threatened [19].
6.6 Operative Treatment
The surgical strategies include bone marrow stimulation, bone grafting, tissue transplantation (autologous or allograft), chondrocyte implantation and prosthesis capping. Salvage options in the case of advanced disease with secondary arthritis include arthrodesis and arthroplasty. Cystic lesions where the cartilage layer over the cyst is well supported and remains intact and attached can be treated with retrograde drilling or retrograde bone grafting [20].
The majority of cystic lesions having primary surgery are <2 cm2, and marrow stimulation techniques should be the first line of surgical treatment. If the cyst is larger than this, is uncontained or is a complex revision case, then another strategy should be attempted, either cartilage transplantation or bone grafting. Some authors have reported good results following repeat marrow stimulation for failed primary surgery, but the success rate is inferior if the lesion is cystic [21].
6.6.1 Arthroscopic Bone Marrow Stimulation
This technique involves breaching the subchondral bone plate multiple times allowing pluripotential stem cells from the resultant bleeding to collect in the lesion (Fig. 6.5). Under the influence of cytokines and growth factors, they differentiate into chondrocyte type cells and produce fibrocartilage containing predominantly type I collagen. Cystic lesions require curettage and penetration of the calcified wall of the cyst. This tissue is mechanically inferior, but the results of the procedure in the short term are generally good with smaller lesion (<1.5 cm2) [16, 22]. The procedure has a low morbidity and complication rate and low cost and is technically undemanding. Gobbi et al. performed a randomised study of 30 patients comparing chondroplasty with microfracture and autologous osteochondral transplantation [23]. At a mean of 53 months, there was no difference in AOFAS scores and single assessment numeric rating. Lee showed good results selecting lesions <1.5 mm2 in patients younger than 50 years. Other authors have found age not to affect the clinical outcome [24]. Short- to medium-term results of marrow stimulation have generally been good [25].


Fig. 6.5
Marrow stimulation with microfracture
Of concern, fibrocartilage has inferior mechanical properties compared to hyaline cartilage, and there is a limit to the size of lesion that will fill with repair tissue. Although Van Bergen et al. showed 74 % good to excellent results at 8–20-year follow-up [26], Ferkel et al. showed a 35 % decline in clinical function over 5 years and a grade of worsening of ankle arthritis [27]. Lee et al. showed at relook arthroscopy in 20 patients that 40 % of lesions were incompletely healed with varying degrees of fibrillation and deterioration in the International Cartilage Repair System (ICRS) grading [28]. Shoulder lesions that are not contained have worse clinical results [29]. There is concern that reparative strategies may be a temporary fix, and we are learning more about the limitations of the procedure.
When appropriate, retrograde drilling allows stimulation of the marrow surrounding the cyst and perforation of the cyst wall without disrupting the intact overlying cartilage. Results seem generally favourable with 88 % of patients treated successfully [30, 31]. This was shown to have superior results compared to transmalleolar drilling by Kono et al. [20] leaving the corresponding plafond cartilage intact.
6.6.2 Excision Curettage and Bone Grafting
In this technique autologous cancellous bone is placed in the cyst after curettage and removal of the sclerotic boarder. The aim is to restore the weight-bearing area of the talus in large lesions and to obtain a fibrocartilage-covering surface. If the overlying cartilage is intact, a part can be lifted creating a trapdoor for curettage and placement of the graft. Autograft is usually harvested from the iliac crest or the ipsilateral tibia. Results of this technique are mixed. Kolker et al. showed on a 46 % failure rate in their small series and cautioned the use of the technique [32]. Contrasting this, Draper et al. showed better clinical results with curettage and bone grafting compared to patients just receiving curettage and microfracture [33]. Patients in the microfracture group had smaller lesions.
6.6.3 Osteochondral Autograft Transfer
Osteochondral autograft transfer involves harvesting single or multiple cylindrical cartilage and subchondral bone grafts from the non-weight-bearing part of the ipsilateral knee and transplanting them into the talar defect after preparation. This technique is not possible when the defect is very large (>3 cm3), as it requires mechanical stability from a press fit of the graft into the talar defect. Shoulder lesions are also more difficult for the above reasons.
Contained talar cysts can be removed and the defect filled with the graft. It is indicated in revision cases when marrow stimulation has not been successful or for primary surgery in cases of contained cystic lesions [16, 34]. The procedure is technically demanding and often requires an osteotomy of the medial malleolus, the fibula or the plafond for access to insert the cylindrical grafts orthogonal to the talus. Congruence of the talar surface is paramount and can be difficult to achieve on the shoulders. Cadaver studies have confirmed increases in contact pressure of the graft if set elevated or increased local pressure on the surrounding cartilage if the graft is recessed too deep [35, 36]. The number of cylinders implanted does not seem to affect the clinical result at 5 years as long as the lesion is well covered by the graft [37].
Cartilage from the knee is thicker than talar cartilage, and the properties of knee cartilage differ from that of the talus. The osseous part of the graft has to sit lower than the bone level in the talus to achieve a flush surface. Donor site knee pain is a concern: Hangody et al. in the largest series of talar mosaicplasty (98) and over 1,000 mosaicplasty cases in other joints had an incidence of long-term knee pain in 3 % of cases [38]. Similarly Kennedy et al. reported an incidence of 4 % [39] but up to 16 % in another series [40]. The number of grafts harvested does not seem to influence the clinical result, but body mass index (BMI) has a negative influence [25]. The procedure provides good outcomes in the short to medium term in upward of 85 % of patients [16]. Replacing the defect with bone and hyaline cartilage and achieving good integration potentially are long-lasting solutions and superior to repeat arthroscopy in cases of failed marrow stimulation [41, 42]. Scranton et al. had a 90 % good to excellent clinical result at a mean of 36 months for cystic, type 5 lesions [43].
The critical size of lesion that dictates whether replacement or repair is the best long-term treatment is yet to be determined. Lesions greater than 8 mm that are more cystic and cases where primary repair has failed are the usual indications [39].
6.6.4 Osteochondral Allograft Transplantation
This replacement procedure transplants viable cartilage and subchondral bone from a fresh cadaver talus to a recipient with a large cystic defect. Some of the advantages of the procedure include the following: (1) It has the ability to match the shoulder of the recipient talus with careful harvesting for uncontained lesions. (2) It can fill massive (>3 cm2) defects that are not amenable to autograft techniques [12, 13]. (3) Tibial or fibula osteotomy is often not necessary for access as the graft can be put in from the anterior approach—one does not have to be orthogonal to the talus as with mosaicplasty or osteochondral autograft transplant [3].
Fresh allograft is preferred over fresh frozen or cryopreserved as chondrocyte activity is optimal [44]. Implantation should take place as soon after harvesting and screening as possible to preserve the living chondrocyte count. This decreases over time to 70 % at 28 days even if the graft is stored in a temperature-controlled environment of 2–4 °C [45].
After excision of the diseased cystic talus, the donor talus is fashioned to fit the defect perfectly and secured with bioabsorbable or recessed permanent screws or pegs (Fig. 6.6).
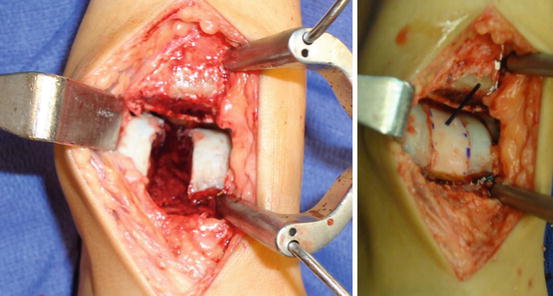

Fig. 6.6
Intra-operative picture of cyst removal and after implantation of the prepared allograft
Although this treatment has its indications and advantages, there is little supporting evidence for the technique. Raikin et al. reported his results of 15 patients at 2-year follow-up with an improvement in the mean AOFAS scores from 38 to 83 and an improvement in pain scores [12]. There were two failures reported (13 %) requiring ankle arthrodesis, and there tended to be radiographic deterioration over time. Urgery et al. showed similar clinical improvement in 38 patients at a mean follow-up time of 37 months. 4 (11 %) patients required ankle arthrodesis for graft failure and arthritis. They performed MRI scans in 15 patients at a mean of 39 months post surgery showing poor graft incorporation and instability in 5 cases with graft-host boarder signal intensity. Ten showed fair to good graft incorporation, and one demonstrated graft subsidence [46].
This joint-preserving procedure should be used in select cases not amenable to marrow stimulation or autograft procedures. It can relieve pain, improve function and fill massive defects prolonging joint function.
6.6.5 Autologous Chondrocyte Implantation
In the quest to replace osteochondral defects with hyaline cartilage, Brittberg et al. first treated lesions in the knee with chondrocytes harvested from non-weight-bearing parts of the knee, cultured and then placed in the defect, covered with a periosteal flap from the ipsilateral tibia [47]. Another second-generation technique has been developed, eliminating the need for periosteal flaps culturing the chondrocytes on a matrix of porcine collagen membrane or hyaluronic acid-based membrane [48]. This can then be placed into the lesion with or without addition of cancellous bone graft and can be performed arthroscopically. Cell distribution is more evenly distributed within the matrix, and dedifferentiation is reduced [49].
Evidence for the technique is sparse in the ankle compared to the knee. Apart from one meta-analysis [50], there are no other level 1 evidence studies. Niemeyer’s analysis identified 16 retrospective studies and 213 cases. The overall clinical success was 89 %, but there were nine different clinical scoring systems used, and the mean study sample size was small at 9 [50]. Giannini et al. in the largest series to date involving 46 patients followed up for 36 months showed improvement in AOFAS scores from 57.2 to 89.5. Three patients had a second-look arthroscopy and biopsy. Macroscopically and histologically the repair tissue was similar to hyaline cartilage [51]. MRI T2 mapping at 24 months post surgery has shown cartilage similar to a normal control group [52]. Both of these studies did not look at large cystic lesions; they were smaller, contained lesions
Results look promising but evidence is lacking compared to research in cartilage repair in the knee. Major limitations of the technique are the need for a second procedure after harvesting cartilage and costs of the procedure. The limit to the size of defect that can be filled by the matrix has not been determined. In these large cystic lesions, there is primarily a bone defect, which this technique will not be able to address.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







