CHAPTER 13 Lymphedema
1. Describe the pathophysiology of lymphedema.
2. Identify and describe two types of lymphedema.
3. Distinguish the difference between lymphedema and lipedema.
4. List two common diagnostic tests used to diagnose lymphedema.
5. Articulate important assessment features for the patient being evaluated for lymphedema.
6. Outline a treatment plan for a patient with lymphedema, including conservative measures and adjunctive therapies such as surgery.
7. Explain prevention and treatment measures for a patient with cellulitis.
Lymphedema is a chronic disease characterized by swelling of the affected body part, usually the limb, because of impaired flow of lymph fluid. Accumulation of lymph fluid results in swelling in the affected limb. The swelling may be mild or it may be severe, such that the individual is unable to use the affected limb. Lymphedema primarily occurs in the subcutaneous fatty tissues of the arms or legs (Lawenda et al, 2009; Sieggreen and Kline, 2004).
The clinical presentation of lymphedema results from the subcutaneous accumulation of fluid. With interstitial fluid accumulation comes an inflammatory response, slowed lymphatic flow, which causes lipogenesis, and fat deposition. Patients develop firmer subcutaneous tissue in the limb as fibrosis as well as hypertrophy of the adipose tissue occur. These physical changes present clinically as soft and pitting edema but later progress to induration and fibrosis with nonpitting edema (Warren et al, 2007b).
Epidemiology
As the third component of the vascular system, the lymphatic system receives the least attention. Similarly, lymphedema and related pathologies have received much less attention than the pathologies associated with venous and arterial ulcers. Chronic lymphedema has no cure, and long-term management with patient involvement is critical to control the lymphedema. Lymphedema is frequently undertreated, and an insufficient number of lymphedema centers and specialists are addressing the problem. Among breast cancer patients undergoing surgery and radiation in the United States, the incidence of lymphedema ranges from 10% to 40%. Globally, 140 to 250 million cases of lymphedema are estimated to exist (Revis, 2008). The incidence of lymphedema is expected to rise as a result of the increasing numbers of obese persons and elderly persons who often have many medical comorbidities but have limited ability to care for themselves as well as limited social and financial resources (Cheville, 2007).
Lymphedema in cancer patients (e.g., breast, gynecologic, colon, bladder cancer; sarcoma) is seen today at more advanced stages of the cancer than in the past because cancer patients are living longer as a result of more effective cancer treatments. With the increased survival of these patients, the numbers of cancer patients with lymphedema is also expected to increase. However, these patients may be sicker and less able to care for themselves, including managing their lymphedema (Cheville, 2007).
Pathophysiology
The lymphatic system is composed of lymphatic vessels and lymphatic tissue, which function as a drainage and transport system. When fluid from the interstitial space enters the lymphatic system, it is considered lymph fluid. Lymph fluid consists of protein, water, fatty acids, salts, white blood cells, microorganisms, and debris. It is absorbed from the interstitial spaces into the lymphatic vessels, where it is transported to the venous system (Holcomb, 2006; Lawenda et al, 2009).
The major function of the lymphatic system is to return fluid and protein from interstitial spaces to the vascular system. Because lymphatic vessels often lack a basement membrane and have thinner vessel walls, they can reabsorb molecules too large for the venous system (Holcomb, 2006; Sieggreen and Kline, 2004). Usually tissue fluid and protein macromolecules filtrated by the arterial capillaries are reabsorbed and returned to the circulation through the lymphatic system. From 50% to 100% of the intravascular proteins are filtered this way in the interstitial space. The lymphatic vessels absorb 2 to 4 L of protein-rich fluid retained in the interstitial space per day. This fluid is picked up by the lymphatic capillaries and returned to the circulation, thus maintaining normal plasma volume and preventing interstitial edema. If the system is overloaded, fluid and protein accumulate in the interstitial space, forming a high-protein edema that triggers an inflammatory response with deposition of collagen (Holcomb, 2006; Macdonald et al, 2003).
Two systems of lymphatic drainage work similarly to the venous system. The superficial system drains the skin and subcutaneous tissues, and the deep system drains the tissues to the fascia and below. The lymphatic vessels in the superficial system are located in the subcutaneous tissues, whereas those of the deep system are aligned with the blood vessels, especially the veins. These two systems of lymphatic drainage are connected by perforating vessels just like the veins (Lawenda et al, 2009; Macdonald et al, 2003).
The lymphatic system is composed of lymphatic capillaries, precollectors, lymph collectors, and lymphatic trunks. Capillaries absorb lymph fluid and transport it to lymph nodes, lymphatic trunks, and lymphatic ducts. The lymphatic capillaries are larger than the blood capillaries, are more permeable with thinner walls, and have no basement membranes allowing them to absorb fluids. The lymphatic capillaries do not have valves, so lymph flows in the direction of the lower pressure. The precollectors connect the lymphatic capillaries with the lymph collectors. The lymph collectors are similar to veins; they have valves and transport lymph fluid to the lymph nodes and lymphatic trunks. The precollectors and collectors are the principal vessels of the lymphatic system and eventually filter through to the lymph nodes (Lawenda et al, 2009; Macdonald et al, 2003).
Lymph drains from the legs into the lumbar lymphatic trunk to the intestinal lymphatic trunk and cisterna chyli to the thoracic duct, which drains into the left subclavian vein (Figure 13-1). The thoracic duct empties approximately 3 L of lymph fluid per day into the venous circulation (Lawenda et al, 2009). From the left side of upper body, the lymphatic vessels of the left arm drain into the left subclavian lymphatic trunk, which then drains into the left subclavian vein. Lymph is drained from the right side of the head, neck, thorax, and right arm into the right subclavian vein by way of the right thoracic duct (Sieggreen and Kline, 2004).
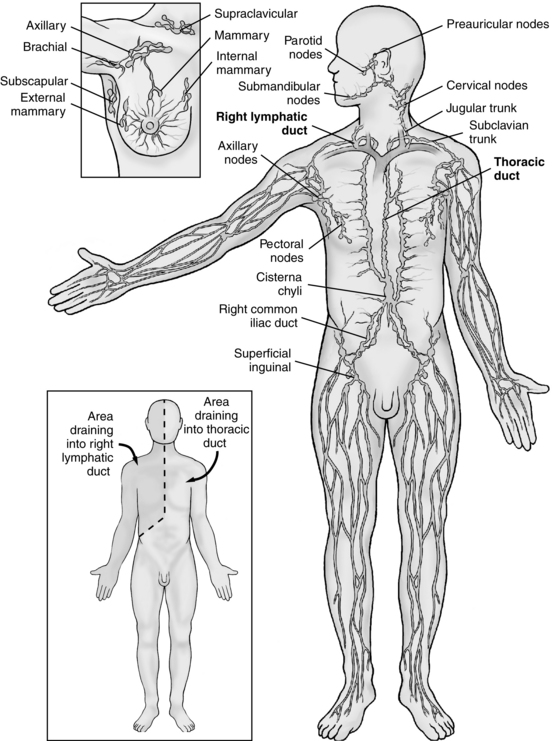
FIGURE 13-1 Anatomy of the lymphatic system.
(From Monahan FD, Neighbors M: Medical surgical nursing: foundations for clinical practice, ed 2, Philadelphia, 1998, WB Saunders.)
Lymphedema is caused by dysfunction of the lymphatic system, where accumulation or pooling of lymph fluid into the interstitial space occurs. The dysfunction is most commonly caused by external forces such as surgery, radiation, or infection, which can damage the lymphatic system. With an impaired lymphatic system, the amount of lymphatic fluid in the interstitial space becomes more than the body’s lymph system can handle. Plasma oncotic pressure decreases, oncotic pressure of tissue fluid increases, and lymphatic blockage or obstruction occurs (Sieggreen and Kline, 2004). With retention of fluid and large protein molecules within this interstitial space, the space swells (Lawenda et al, 2009). With swelling, the large protein molecules leak into the tissues, causing fibrosis. Over time progressive obstruction to lymphatic flow occurs by distortion or obliteration of the lymphatic channels from the fibrotic changes in the tissues (Macdonald et al, 2003; Sarvis, 2003; Tiwari et al, 2003).
Types of lymphedema
Lymphedema may be acute or chronic. Acute lymphedema is a condition that lasts less than 6 months and is associated with pitting edema with pressure and lack of brawny skin changes. Risk factors for acute lymphedema include surgical drains with extravasation into the surgical site, acute injury to the limb, radiation therapy, infection, and phlebitis (Sieggreen and Kline, 2004). Chronic lymphedema, unlike acute lymphedema, is not reversible and requires lifetime management. Lymphedema may be classified as primary (idiopathic) or secondary (acquired) (Holcomb, 2006).
Primary (idiopathic) lymphedema
Primary lymphedema is caused by congenital absence or abnormalities of lymphatic tissue. It affects between one and two million individuals in the United States and usually involves the leg (Holcomb, 2006; Revis, 2008). Primary lymphedema may be classified by time of onset: congenital lymphedema is detected at less than 1 year of age, lymphedema praecox is detected from ages 1 to 35 years, and lymphedema tarda is detected after age 35 years (Holcomb, 2006; Kerchner et al, 2008). The congenital type may be sporadic or familial. The familial form is called Milroy disease and is thought to be linked to autosomal inheritance of a mutated gene. Congenital lymphedema affects both lower extremities but can involve the upper extremities as well as the face. The most common type of primary lymphedema is lymphedema praecox (Meige disease). It usually affects adolescent women. Meige disease occurs when some lymphatics that are present at birth become damaged by infection or when the number of lymphatics is inadequate as the individual grows and matures. The condition usually is unilateral, involving the foot and calf. Lymphedema tarda is seen in individuals after age 35 years who have congenitally weakened lymphatics so that a precipitating event such as injury can result in lymphedema (Kerchner et al, 2008).
Secondary (acquired) lymphedema
Secondary lymphedema affects between two and three million individuals in the United States (Holcomb, 2006). The most common cause of secondary lymphedema in the United States is related to malignancy and its subsequent treatment. The types of cancers associated with secondary lymphedema include breast cancer, gynecologic cancer, lymphoma, melanoma, and urologic cancers. Secondary lymphedema is caused by dysfunction or obstruction of the lymphatic system that usually occurs at proximal limb segments (i.e., lymph nodes) due to surgery, radiation, trauma, infection, malignancy, or scar tissue. Surgery may include the removal of one or more lymph nodes. If the remaining lymph nodes and vessels cannot compensate for those that have been removed, then lymphedema can result. The current use of sentinel node biopsy (e.g., with cancer surgery) has helped reduce the risk of patients developing lymphedema because fewer lymph nodes are removed. Radiation can cause scarring and inflammation of the lymph system, which can restrict the flow of lymph fluid, thus increasing the risk for lymphedema. Cancer can block the lymphatic vessels, which also increases the risk for lymphedema.
Infection from parasites can infiltrate the lymph vessels and block the flow. The cause of the most common form of infection, called filariasis, is the nematode Wuchereria bancrofti, which is transmitted to humans by mosquitoes. When the filarial larvae mature into adult worms in the lymphatic channels, they block the lymph channels, causing severe lymphedema in the arms, legs, and genitalia, a condition also known as elephantiasis. It infects more than 90 million people globally, occurs mainly in southeast Asia, India, and Africa, and is the most common cause of lymphedema worldwide (Macdonald et al, 2003; Tiwari et al, 2003).
Once damage has occurred to the lymphatic system, transport capacity is permanently decreased predisposing to lymphedema. The pelvic and inguinal nodes in the lower limbs and the axillary nodes of the upper limbs are the primary sites of obstruction (Sieggreen and Kline, 2004). Lymphedema has been reported 20 to 30 years after the precipitating event (e.g., surgery).
With the rise in obesity rates comes an increase in the number of cases of secondary lymphedema in the morbidly obese. In a review of wound clinic data of approximately 15,000 patients from 17 wound centers in the United States, Fife and Carter (2008) found 74% prevalence of secondary lymphedema in morbidly obese patients. Morbidly obese patients also have been observed to have lymphedema in conjunction with venous ulcer disease. Obesity impedes lymphatic flow, leading to accumulation of lymphatic fluid in the subcutaneous tissue (Kerchner et al, 2008). The risk for lymphedema increases as body mass index increases (Dell and Doll, 2006). Sizable weight gain has been shown to increase a woman’s risk for lymphedema following breast cancer (Warren et al, 2007b). With the increase of lymphatic fluid in the setting of decreased oxygen tensions, fibrosis with chronic inflammation and increased risk for infection result. In addition to occurring in the extremities, lymphedema can be seen in the overhanging abdominal pannus of the morbidly obese (Kerchner et al, 2008).
Stages of lymphedema
The International Society of Lymphology recognizes four categories or classifications for staging (Table 13-1). Stage 0 is a subclinical lymphedema in which swelling is not evident despite impaired lymphatic function. Stage 0 (reversible) may exist for months or years before edema occurs. Stage I (reversible) is defined as lymph fluid with a high protein content (in contrast to venous ulcer disease edema) that dissipates after limb elevation. Pitting may occur. Pitting edema is considered present when, 5 seconds after a finger is pressed into the edematous tissue, an indentation remains. In stage II (irreversible), pitting edema is present but limb elevation alone does not reduce edema. As stage II progresses, tissue fibrosis develops, and pitting may or may not be present. Stage III (irreversible) is elephantiasis in which pitting is not present but skin changes in the limb, such as acanthosis (increase in thickness of the epidermis), fatty deposits, and warty growths, may be present. Within each stage, limb volume differences can be categorized as minimal (<20% increase in size), moderate (20%–40% increase in size), and severe (>40% increase in size) (Holcomb, 2006; Macdonald et al, 2003).
TABLE 13-1 Stages of Lymphedema
| Stage | Manifestations |
|---|---|
| 0 | Subclinical lymphedema with edema that is not evident despite impaired lymphatic function |
| I | Reversible pitting edema that begins distally (at foot) Negative or borderline Stemmer sign No palpable fibrosis |
| II | Minimally pitting or nonpitting (brawny) edema that is not reduced by conservative measures such as elevation Positive Stemmer sign Pronounced fibrosis Hyperkeratosis (thickening of skin) Papillomatosis (skin has rough cobblestone appearance and texture) |
| III | Lymphostatic elephantiasis (massive enlargement and distortion of limb caused by breakdown of skin’s elastic components) Progressive fibrosis, acanthosis, hyperkeratosis, papillomatosis Ulceration |
Data from the International Society of Lymphology, The diagnosis and treatment of peripheral lymphoma: 2009 Consensus Document, Lymphology 42(2):51-60, 2009, available at http://www.lymphnotes.com, accessed April 14, 2009.
Lipedema
Lipedema, often confused with lymphedema, is a syndrome of bilateral adipose deposition that almost always occurs in overweight women. Lipedema usually presents around puberty with a familial tendency of enlarged or fatty legs and buttocks (hips and thighs). Features distinguishing lymphedema from lipedema are listed in Table 13-2 (Fonder et al, 2007; Warren et al, 2007a). Table 10-1 compares characteristics of venous edema to lipedema and lymphedema.
TABLE 13-2 Comparison of Lymphedema and Lipedema
| Lymphedema | Lipedema | |
|---|---|---|
| Gender | Male and female | Female |
| Age | Any age | After or during puberty |
| Edema | Pitting progresses to firm fibrotic | Nonpitting |
| Epidermal skin changes | Common | Uncommon |
| Cellulitis | Common | Uncommon |
| Stemmer sign | Positive | Negative |
| Distribution | Unilateral or bilateral, toes to groin | Always bilateral, ends at ankles |
| Tenderness | None | Tender to palpation Bruising common |
| Magnetic resonance imaging | Honeycomb pattern | Normal |
The diagnosis of lipedema usually is based on history and physical examination. Diagnostic tests, such as those done with lymphedema, may be conducted and usually only show subcutaneous fat hypertrophy. Treatment options for lipedema are mainly dietary and lifestyle modifications. However, research now is assessing the role of liposuction in treating lipedema patients. Schmeller and Meier-Vollrath (2006) treated 28 female patients with liposuction. Twenty-one patients were reevaluated after an average of 12 months. All patients showed an improvement in body proportions. Eighteen patients suffered from pain before the procedure; 10 patients had improvement in pain levels after liposuction. All patients complained of sensitivity to pressure. After the liposuction, this symptom disappeared in 8 patients and improved in 13 patients. Generally lipedema responds poorly to compression therapy and leg elevation, although low-level compression therapy usually is recommended to prevent the disease from progressing to lipolymphedema, which is a combination of lipedema and lymphedema (Fonder et al, 2007; Kerchner et al, 2008; Schmeller and Meier-Vollrath, 2006).









