Fig. 7.1
Factors influencing surgical outcome of TJA
Obesity
Association with Osteoarthritis
Obesity nowadays has become a major health issue. The prevalence of obesity nearly doubled from 1980 to 2008 (6.4–12 %) with half of this rise taking place in the last decade. Currently, more than 500 million patients worldwide are considered to be obese. In 2009–2010, the age adjusted prevalence of obesity was 35.5 % among adult men and 35.8 % among adult women in the U.S. [5, 6]. The development of osteoarthritis has been strongly correlated with obesity by implicating the excessive mechanical load exerted on the knee cartilage as a causative factor. Increased body weight has been found to be more destructive for the knee rather than the hip joint and in relation to the acceptable BMI(<25), there is multiple times increase of possibility for TKA with every increase in the body mass index scale [7–13]. Fehring et al. have suggested that the osteoarthritis risk is fourfold for obese men and fivefold for obese women [14]. It has also been suggested that in patients with a BMI >35, TKA may be required almost 8 years earlier than for those who maintain a normal BMI <25 [15], while another study has shown that morbidly obese patients with gonarthrosis will require TKA 13 years earlier than those with normal BMI [16]. On the other hand, a decrease of 5 kg of body weight (body mass index of 2 units or more) has been shown to decrease the risk of osteoarthritis in women by at least 50 % [17]. Alternatively, 24 % of surgical interventions might be avoided if patients reduce their weight by 5 kg or until their BMI reaches proposed normal levels [13]. During the single leg stance of gait cycle, a force of three to six times of body weight is transmitted across the joint. A study by Messier, revealed a statistically significant direct association between body mass and peak values of compressive forces, resultant forces, abduction moment, and the medial rotation moment of the knee. Each weight loss unit results in a fourfold reduction in the load exerted on the knee per step during daily activities [18]. Biomechanical factors are also considered to be causative factors in the development of knee osteoarthritis. The presence of mechanoreceptors at the surface of chondrocytes, which are activated by increased pressure, induce cartilage degradation by the production of proinflammatory mediators, such as nitric oxide and prostaglandin E2. Proteins produced by the adipose tissue, the adipokines (especially leptin), have also been implicated in the cartilage destruction process. Adipokines along with other cytokines are responsible for the production of nitric oxide; this interferes with chondrocyte function resulting in the loss of cartilage matrix through induction of apoptosis, activation of metalloproteinases, and inhibition of proteoglycan and type II collagen synthesis [19, 20].
Early Complications
Obesity is defined as an abnormal or excessive accumulation of fat on the human body leading to increased health problems that may reduce life expectancy. Diabetes mellitus, coronary artery disease, hyperlipidemia, hypertension and obstructive sleep apnea are the medical co-morbidities that usually accompany obesity. These comorbidities are theoretically the reason for the higher perioperative complication rates in obese patients. Venous thromboembolism is strongly correlated with obesity and delayed postoperative ambulation, and has been a major concern for the orthopedic community for many years, with several studies being published giving postoperative guidelines for antithrombotic medication prophylaxis. Sixty to 90 % of patients with OSAS (obstructive sleep apnea syndrome) are obese and postoperative respiratory and cardiac complications and length of hospital stay after joint replacement are significantly higher compared to non-obese patients [21]. Anesthesiologists may often be unable to perform spinal anesthesia in obese patients because of excessive body fat, leading to the use of general anesthesia, thus increasing the risk of immediate postoperative respiratory complications (Fig. 7.2). In cases of extreme obesity, the perils of postoperative in-hospital complications are increased 8.44 times, while the odds ratio for length of stay, outpatient complications and readmission rates for every 5 units of BMI >45 are multiplied [22]. These parameters contribute to the increased cost of health care provision.

Fig. 7.2
Performing spinal anesthesia on the obese patient
Classification
Body mass index (BMI) is the calculating tool commonly used to determine and classify categories of severity of obesity in adults (Table 7.1). Obesity is generally defined as a BMI >30 kg/m2. BMI is calculated according to the height and weight of an individual using the formula: BMI = weight (kg)/height2 (m2) or = [weight (pounds)/height2 (inches2)] × 703. Even though BMI is the measurement of choice for most health care providers, for many years its validity has been questioned, as if its longevity, having been developed 150 years ago, somehow discredits it. The main concerns are that it does not take into account age or gender and that it cannot distinguish weight associated with muscle mass compared with increased adipose tissue. Newer index screening tools have being developed in order to more accurately assess body fat, such as the body adiposity index (BAI) and the waist-hip ratio (WHR) [23–25]. Nevertheless, BMI still remains an inexpensive tool, which is widely and currently accepted because of its simplicity, for data accumulation.
Table 7.1
Categories of severity of obesity are shown
BMI (kg/m2) | Classification | Risk of comorbidities |
|---|---|---|
<18,5 | Underweight | Low |
18,5–24,99 | Normal weight | Average |
25–29,99 | Overweight – preobese | Increased |
30–34,99 | Obese (obesity I) | Moderate |
35–39,99 | Severe obese (obesity II) | Severe |
40–49,99 | Morbidly-obese | Very severe |
>50 | Super-obese | Very severe |
>60 | Super-mega-obese | Very severe |
Long Term Outcomes
Many studies have tried to define the relationship of obesity with TKA regarding postsurgical complications. Wound healing problems, superficial infections, deep infections leading to removal of the prosthesis, fusion or amputation, component malposition and aseptic loosening are all significant related problems that the orthopedic surgeon has to deal with.
Periprosthetic Joint Infection
The association of obesity to periprosthetic joint infection has already been established, with morbidly obese patients showing the highest increase in complication rates. Jamsen et al. [26] revealed an increase in infection rate from 0.37 to 4.66 % in this study group compared with patients with normal BMI in the first postoperative year, while Maliznak et al. [27] found an increased odds ratio of infection of 21.3. Winiarsky et al. [28] reviewing 50 cemented TKA in morbidly obese patients, highlighted the problem of the increased risk of poor wound-healing, infection and avulsion of the medial collateral ligament in this study group. Many other studies with large groups of patients have come to the conclusion that with an increasing BMI, and especially BMI >40 with the presence of diabetes, the probability of periprosthetic joint infection is multiplied [29–34].
Component Malposition
Accurate component alignment plays an important role in the load distribution between knee compartments. Full-length standing hip to ankle X-rays are needed to determine the correct mechanical axis and classify the total knee replacement as aligned or mal-aligned. Precise reconstruction with 0° ± 3° deviation from the mechanical axis, even though other factors may also be important, would result in decreased stresses across the joint line, limiting polyethylene wear and enhancing implant durability [35, 36]. The detrimental effect of obesity in postoperative TKA limb alignment has been identified [37, 38]. Setting up the patient on the operating table and placing the tourniquet is always difficult and time-consuming and should be done with extreme caution, in such a way that surgical operation will not be compromised, especially by tourniquet loosening (Figs. 7.3 and 7.4). In morbidly obese patients especially, thick subcutaneous tissue makes surgical exposure of the joint difficult. Longer mid-lined incisions, eversion of the patella, and increased tourniquet length and width may be required (Figs. 7.5 and 7.6). Limited vision and the difficulty in accurately positioning the cutting guides may result in a non-successful surgical operation with a poor long-term outcome for the patient [39]. Greater traction of the soft tissue envelope in order to achieve better visualization, longer operating time, poor vascularization of fatty tissue and reduced immune response are all factors that may increase infection rate by 6.7 times in patients with BMI >35 [40]. An anthropometric study in 2008 in severely and morbidly obese patients has attempted to identify those who pose greater surgical difficulties during TKA by proposing new indexes. A suprapatellar index (length of the extremity to be operated on with the circumference of the knee above the upper pole of the patella) below 1.6 is associated with greater surgical difficulty [41]. The femoral and tibial component, when using a femoral intramedullary guide and an extramedullary tibial guide, has the tendency to align in a more varus position in a morbidly obese group [femoral component: 5.0° vs. 6.5° valgus (P < 0.05), tibial component: 2.5° vs. 1.0° varus (P < 0.05)] [42]. The use of an intramedullary tibial cutting guide makes surgical intervention easier by maintaining the anatomical axis of the tibia as a reference point for correct orientation and reduces operating time [43]. Some authors advocate the use of implant designs with minimal constraint such as posterior stabilized or in cases of severe instability a rotating hinge implant in order to achieve minimal constraint in morbidly obese patients [44]. They also advocate the use of implants with short tibial rods, thus increasing the contact area and reducing the load transferred under the tibial plate and, more recently, the use of trabecular metal tibial components which provide earlier anchoring to the bone [45].
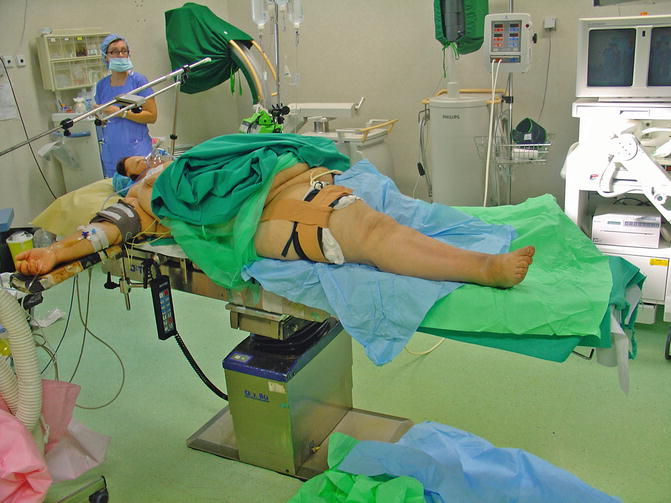
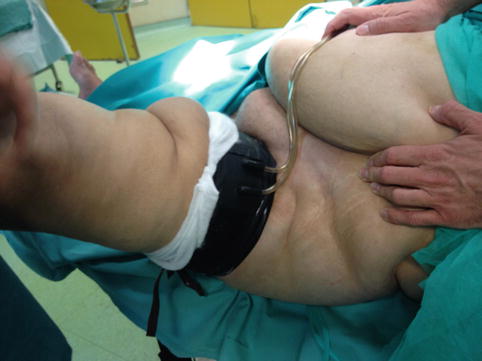
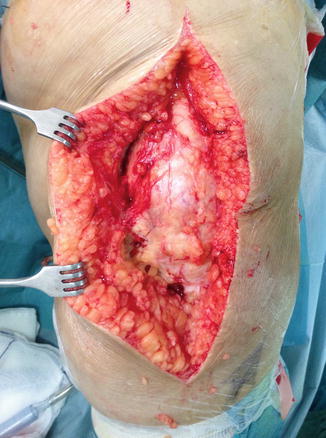
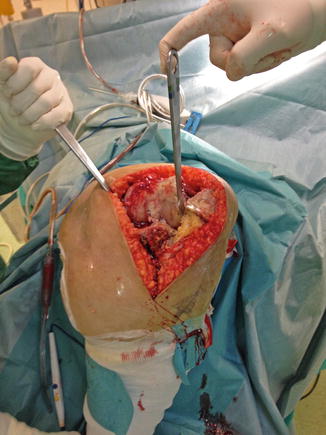

Fig. 7.3
Positioning and proper tourniquet placement is difficult but essential for the success of the operation

Fig. 7.4
Positioning and proper tourniquet placement is difficult but essential for the success of the operation

Fig. 7.5
Thick subcutaneous tissues with extended midline incision needing greater traction and patella eversion

Fig. 7.6
Thick subcutaneous tissues with extended midline incision needing greater traction and patella eversion
Aseptic Loosening
Obesity has been implicated in aseptic loosening of the TKA components, a phenomenon that can be confirmed by the existence of radiolucent lines in the bone-implant interface. Spicer et al. [46] compared the clinical and radiographic results of 326 TKAs in patients with BMI >30 and 425 TKAs in patients with BMI <30. Although 10 year implant survivorship was similar and linear osteolysis was comparable, focal osteolysis rates were five times increased in the morbidly obese group [46]. Dewan et al. [47] compared 220 cemented tricompartmental TKAs (41 knees in patients with BMI >40) with a mean follow-up of 5.4 years and concluded that morbidly obese patients are 5.4 times more likely to develop patellar radiolucency, have poorer hamstring and quadriceps strength while more patellofemoral problems are encountered. During flexion, the force exerted on the patellofemoral joint is three times the body weight. In increased BMIs, the bigger stresses across the prosthetic joint may reach the threshold for starting patellar radiolucent lines to appear sooner. Studies have shown that the patella attaches to the intercondylar notch of the femoral component at 90–105°, depending on prosthesis design, reaching its highest peak of contact stresses. A decreased deep flexion which is observed in obese patients due to the mechanical stop caused by the greater amount of adipose tissue may enhance stresses responsible for producing patellofemoral symptoms, making ROM even more difficult beyond this point [47]. However, Cavaignac et al. [48] studied 212 unicompartmental knee arthroplasties and found that 10-year survival rates were almost similar for patients with BMI >30 and BMI <30 (92 % vs 94 %) [48].
Weight Loss After TKA
Obesity and physical activity seem to be strongly correlated after TKA. There is a belief that decreased physical activity because of pain postoperatively will increase body weight. Booth showed in 2002 that only 18 % of obese people lose weight after joint replacement [39]. In 2005 Heisel, investigating weight change in 100 TJA, concluded that neither patients with normal BMI nor obese patients lost weight, while merely overweight patients gained a significant amount. Obesity should thus be treated as an independent disease that is not the result of inactivity due to arthritis [49]. Dowsey et al. in 2010 investigated 529 TKAs at 1 year postoperatively found a clinically significant weight loss of 5 % in 40 (12.6 %) of obese patients, while 107 (21 %) had gained weight [50].
Functional Outcome
Conflicting results emerge from the literature, when comparing functional outcome in obese and non-obese patients (Figs. 7.7 and 7.8). There is a need for better and more organized studies that distinguish the various BMI categories which would generate more comparable data to help reach a general consensus concerning the relationship of obesity and functional outcome. This is especially true for categories of BMI >30 and BMI <40, while data for morbidly obese patients are more conclusive, leading orthopedic surgeons to recommend the loss of excess BMI in these individuals for a better long term outcome. Stickles et al. [51] investigated the outcome of 1,011 TKAs 1 year postoperatively by measuring WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) and including the SF-36, and concluded that there were no differences between obese and non-obese patients regarding satisfaction and the decision to repeat surgery, but WOMAC scores were lower for those with higher BMI. Also, increased body mass index was associated with more difficulty in ascending or descending stairs at 1 year [51]. In 2012 Colllins et al. [52] published their work on 445 consecutive primary TKAs prospectively followed up to 9 years comparing the clinical outcomes between non-obese and obese (BMI >30) patients. All groups showed great improvements with no significant differences in revision or implant survival rate after 9 years of follow-up. However, lower function scores were seen at all follow-up periods prior to 9 years in the highly obese subset (BMI >35) [52]. In a recent study of 535 cementless TKAs with a mean follow-up of 9 years Jackson et al. [53] found significantly lower HSS scores in obese individuals. In two studies published in 2004 Foran compared TKA outcomes between obese and non-obese patients. In the first study, the author analyzed 78 TKAs performed on obese patients over a 7 year period and found that this group had worse functional outcomes (measured according to the Knee Society Score) than non-obese controls, with the morbidly obese subgroup maintaining the lowest scores [54]. In the second study, the author conducted a 15-year follow-up of 30 non-cemented knee implants in matched case and control groups with no preoperative differences in functional status. At the end of follow-up the non-obese patients had a better Knee Society objective Score (89 vs. 81 in obese patients) [55]. Two studies by Núñez in 2007 [56] and 2009 [57] analysed the factors associated with worse functional outcomes following total knee arthroplasty. Follow-up over 3 and 7 years showed that WOMAC scores, especially on the pain scale, were worse among severely obese patients (BMI >35 kg/m2) than in non-obese individuals. McElroy et al. [58], in a systematic review, concluded that a BMI >40 at a mean of 5-year follow-up, is associated with lower Knee Society Objective and Function Scores, lower implant survivorship and higher complication rates compared with patients with a BMI within normal range [58]. On the other hand, there are other investigations that do not find significant differences between obese and non-obese patients regarding the outcome of TKA. Singh et al. [59] found that there is no association between BMI and moderate-severe pain in a 5-year primary TKA, implying that obese patients should not be discouraged from total knee replacement. Hamoui et al. [60] compared 30 TKAs in 21 obese patients with 53 TKAs in 41 non-obese patients with a median follow-up of 11.3 years and concluded that KSS scores, osteolysis, radiolucency and revision rates were not statistically significant between these groups. In an even more recent study, 13,673 patients from the British National Joint Registry were assessed according to reported outcome related to TKA using the Oxford Knee Score and general health EuroQol 5D questionnaires. The improvements in patient reported outcome measures were similar, irrespective of BMI, although wound complications were significantly higher at a rate of 17 % in patients with a BMI between 40 and 60 [61]. Issa et al. [62] compared 210 knees in 174 obese patients (BMI >30) with a non-obese control group. There were no differences regarding implant survivorship (98.8 % vs 98.6 %) and the mean postoperative Knee Society Objective and Function scores (90 and 87 points vs 91 and 89 points). However, obese patients had higher complication rates (10.5 % vs 3.8 %) and achieved a significantly lower mean postoperative UCLA activity score [62].
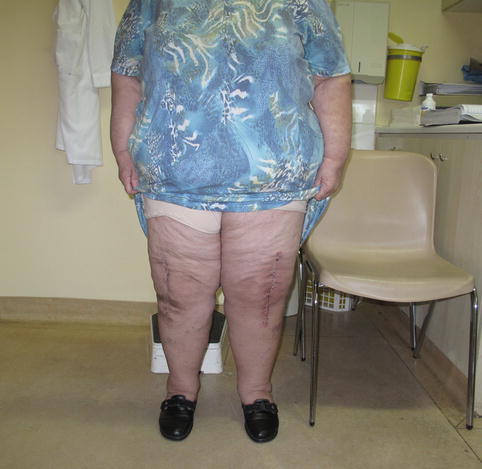
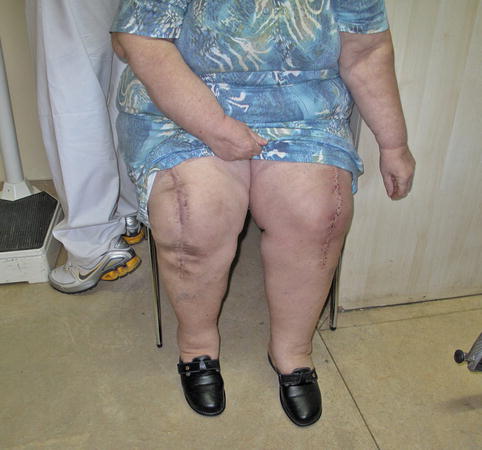

Fig. 7.7
Bilateral TKA in morbidly obese patient with good functional outcome and knee flexion >90° on the right side

Fig. 7.8
Bilateral TKA in morbidly obese patient with good functional outcome and knee flexion >90° on the right side
Level of Activity
The level of activity after total knee arthroplasty is associated with patient specific factors such as age, body mass index, gender, presence of other joint replacement and comorbidities. The outcome is usually determined by pain, function and satisfaction and a standardized rating system has been developed for this purpose. The Knee Society (modified) clinical rating system and the University of California at Los Angeles (UCLA) activity rating scale are the ones mainly used.
Biomechanics
Factors influencing polyethylene wear are material properties, thickness of the component, length of stay, load transmitted and contact area. Repeated high loads and cyclic stressing may cause PE fatigue failure leading to earlier wear. The load is highly dependent on body mass index and level of activity. Biomechanical studies have shown that transmitted compressive forces through the tibiofemoral component are: for downhill walking eight times, stair descent six times, and level walking 3.5 times the Body Weight. Slow jogging is eight to nine times and fast jogging more than ten times the Body Weight [63]. The knee is flexed more during the stance phase of jogging (15–45°) than during walking (5–25°) resulting in a fivefold increase in the flexion moment around the knee, making jogging a high-impact force generally not recommended as a post TKA exercise [64].
Age and Activity Level
Implant durability in correlation with patient’s age and level of activity has been for many years a matter of discussion, with conflicting data in the literature. Younger patients have been considered to be more active with increased life expectancy, which in turn may lead to high mechanical joint loads and earlier polyethylene wear, component breakage and prosthetic loosening. Implant failure is usually addressed via a revision procedure. There are studies with a small number of subjects younger than 55 years treated with TKA that show an implant survival rate of more than 96 % at 10 years and 87 % at 18 years [65–67]. In a meta-analysis of 13 studies Keeney et al. [68] calculated the initial 6–10 year survival rates as 90.6−99 % and the 15 year survival rates as 85–96.5 % in patients <55 years of age. Heyse et al. [69] reported that the 10 year survivorship of TKA was 95 % and the 20 year survivorship 82 % in patients with juvenile idiopathic arthritis (mean age 30 years). Whether the implant is cemented or cementless does not seem to affect the final result, with femoral component survival rate of 100 % at 17 years, and tibial component survival rate of 100 and 98.7 % respectively at 17 years [70]. UKAs seem to have a better outcome in younger patients with estimated survivorship of 96.5 % at 10 years [71]. Scores of satisfaction, range of motion and ability to kneel in patients under the age of 55 treated with UKA are also higher than those treated with TKA, while the opposite occurs at ages over 65 [72]. Mont compared high and low activity patients at a minimum follow-up of 4 years (mean 7 years) and found no effect of low to moderate impact sports regarding overall satisfaction, rate of revision and clinical and radiographic results [73]. On the contrary, a study by Kim et al. [74] suggested that younger age seems to be the major determinant in polyethylene wear, while infection and aseptic loosening remain secondary causes. Meehan et al. [75] concluded that patients younger than 50 years old had almost 2.5 times (1.36 % vs 3.49) greater risk of mechanical failure rather than periprosthetic infection, and 4.7 times higher revision rates due to aseptic loosening compared with patients older than 65 years of age. Julin et al. [76] evaluated 32,019 TKAs from the Finnish Arthroplasty Register and concluded that after 5 years the implant survival rate was 92 % for patients aged <55 compared to 97 % in patients >65 years of age. Harrysson et al. [77] analyzed revision rates in 21,761 patients older than 60 years and 1,434 patients younger than 60 years and the cumulative revision rates at 8.5 years were almost double in the younger group (13 % vs 6 %). Dy et al. [78] evaluated 310,995 TKAs at 10 years postoperatively and concluded that patients 50–75 years of age had a lower revision rate than patients younger than 50 years (hazard ratio 0.47). Lavernia et al. [79] studied 28 autopsy retrieved polyethylene specimens and suggested that more creep and deformation was strongly associated with increased levels of activity. Kurtz et al. [80] projected the demand for primary and revision TKR in 2030 and concluded that in 340,000 revisions by that time 62 % will be done in the age group less than 65 years. In any case, though, the survivorship of the prosthetic design should be based on patient functional performance rather than age, because younger age does not always correlate with high activity levels [81]. In order to achieve implant durability, constant progress is being made in the improvement of bearing materials and surgical techniques in order to lower polyethylene stresses. For this purpose cross-linking PE, improved femoral component surface finish, better modular tibial locking mechanisms, and the use of mobile bearing TKA designs have been proposed as materials and designs that will reduce contact stresses and loads transmitted to the fixation interface [82].
Work Activities
Many patients undergoing TKA, especially the younger and more active (less than 60 years old), look forward to starting or returning to pre-surgery activities. Employment positively affects patient’s mental health by increasing individual satisfaction and maintaining the sense of fulfillment and purpose. The time to return to work depends on the physical demands of the patient’s job. The recovery time for lighter jobs averages 7 weeks and for heavier, demanding jobs can reach 11 weeks. After 1–3 years with a TKA, the percentage of patients with more demanding jobs still working reaches 91 % [83].
Athletic Activities
Primary and revision total knee replacement have been shown to be extremely effective in pain relief, the key element that enables the elderly to participate in sport and daily living activities, so that they can maintain good health and perhaps prolong life expectancy [84]. Functional outcome is defined as the range of motion, with a minimum of 90° flexion being essential for common daily activities such as climbing and descending stairs and rising from a chair. Variables that affect ROM are preoperative knee flexion, diagnosis, BMI, age, surgical technique, implant design and rehabilitation. Kneeling and squatting seem to be the most difficult activities for osteoarthritic knees and postsurgical improvement in this parameter can boost patient satisfaction [85–87]. The literature shows that unicompartmental knee arthroplasty has better results in terms of kneeling ability when compared with TKA or patellofemoral replacement (PFR) [88]. A study by Ries showed improvement of cardiovascular fitness, with increase in the duration of exercise and maximum workload, in patients undergoing total knee arthroplasty compared with a non-surgically treated control group [89]. Certainly, there are some rules that patients should follow in order to have a better and longer-lasting outcome. The risk of wear and osteolysis leading to implant failure and aseptic loosening in higher activity levels and high impact sports should be noted. Painless range of motion with good quadriceps and hamstrings muscle function must be restored before participation in sports in order to avoid possible injuries. Table 7.2 shows the recommended sporting activities as described by Swanson et al. [90]. Generally, there is a consensus of more than 95 % among surgeons who recommend (without limitation) walking on even surfaces, climbing stairs, bicycling on even surfaces, swimming and golfing for both THA and TKA. On the contrary, jogging, sprinting and skiing on difficult terrains are strongly discouraged. Nevertheless, there is more flexibility for restrictions in THA concerning walking up stairs, jogging or playing tennis [90]. Even though studies in the literature have shown promising results for participation in athletics after THA, the outcomes after TKA are not so encouraging. Brander et al. [91] showed at a mean follow-up of 25 months in subjects 80 years or older that the ability to walk five blocks increased from 2 to 50 %, while Zahiri et al. [92] showed that walking activity level decreases by 34 % in patients >60 years old. Huch et al. [93] analyzed all sport activities preoperatively and 5 years after TKA in 300 patients with age less than 74 years and found a decrease of 8 %, a percentage that can be explained by increasing age, pain in the operated knee, surgeon’s instructions to avoid vigorous activities and patient’s rejection of the artificial joint. In the same study, more than 16 % of patients with TKA reported pain at follow-up [93]. Dahm et al. [94] reviewed 1,206 patients who underwent primary TKA with the PFC or Sigma posterior stabilized (DePuy) implant at a mean of 5.7 years (99 % of them with patella resurfacing) and found that 91 % reported satisfaction with their activity level while differences between men and women and age were not statistically significant. Bradbury et al. [95] reviewed 208 TKAs, with 199 of them being uncemented, 5 years after surgery. Patients who participated in sports prior to surgery increased their athletic activities by 65 %. He also found that returning to low impact activities such as bowling was more likely (91 %) than returning to high impact activities such as tennis (20 %). Trying to compare unilateral versus simultaneous bilateral knee arthroplasties, even though the low number of cases (20) may not be sufficient to draw a conclusion, he found that 75 % returned to previous sports activities. Pain relief from both operated knees may be one explanation [95]. Hopper et al. [96] compared 76 total versus 34 unicompartmental knee arthroplasties, according to participation in low-impact athletic activities, preoperatively and 22 months postoperatively. From the 55 and 30 sport participants in the TKA and UKA group accordingly, only 35 (64 %) in the TKA and 29 (97 %) in the UKA group continued their sporting activities. It also seemed that patients with UKA returned earlier to sport and felt that surgery increased their sporting abilities [96]. Another study, reported by Naal et al. [97], on 77 unicompartmental arthroplasties using the Preservation prosthesis (DePuy), found that 73 (95 %) of patients returned to pre-surgery athletic activities, mainly hiking, cycling and swimming, with almost 70 % of them returning less than 6 months from the operation.
Table 7.2




Recommended sport activities for patients with a TKA
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







