Fig. 12.1
(a) Demonstration of pre-operative planning on MRI of needle placement for a tumor on the anterior side of the tibia using conservative (cortical bone) treatment ranges. Three probe placements were needed for this tumor. (b) Per-operative workflow. The CAS screen on the left is in target mode for one of the probe locations. The surgeons are locating this planned position using the pointer tool. The iso-c screen in the back shows the intra-operative CT-scan that was used for CAS matching and MRI/CT fusion. (c) The probe in situ during a run. The patient tracker is visible proximally of the probe. The iso-c arm (left) and CAS (right) are in the back. Temperature of the skin, cooled using wet gauzes is monitored using an infrared thermometer
Workflow:
1.
Fiducials or patient trackers are placed for patient tracking. In the OR this can be done under fluoroscopic guidance, placing two rigid pins in an anatomically safe location. Generally navigation accuracy using trackers is better than with fiducials.
2.
System set-up, the registration and check of instruments and patient tracker
3.
An acquisition scan is performed
4.
Fusion with the planning data/the planning is created.
5.
Real-time guidance of the instruments using navigated drilling-guides or drills
With the expanding use of local ablation in metastasis surgery it can be used as a step in an open procedure for example for stabilization of highly vascularize metastatic lesions. In this case a normal image based setup procedure can be performed using standard landmark and surface matching.
Post-operative control scans after local ablation can best be performed using MRI. MRI T1 and T2 sequences without contrast clearly show the halo, the ablated area where dead tissue resides. Figure 12.2 demonstrates the halo on a post-operative MRI scan after RFA ablation.
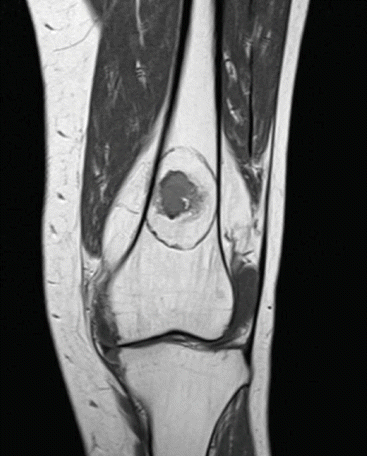

Fig. 12.2
Post-operative MRI slice (T1-TSE) of an ablated atypical cartilaginous tumor clearly shows a halo sign around the lesion
While navigation provides orientational guidance of the probe, the radiologist or the surgeon still has to accurately place the needle. With the development of robotic systems both orientation and execution can be performed by the computer. The first studies comparing robotic to standard CT-guided needle placement in soft tissue find a quicker needle placement time, good accuracy and slightly lower mean ionizing radiation use [12–14]. However, most papers describe robots that work with real-time CT navigation or a feedback loop of imaging and adjusting, this because of the limitations of soft tissue navigation. Decreasing procedural radiation exposure is hard using this workflow. Currently, as far as we know, no papers have been published on robotic guided needle placements in bone.
MRI guidance requires specialized non-magnetic equipment and is still expensive. Furthermore due to interference RFA treatment and image acquisition cannot be performed at the same time. MR thermometry sequences are available and can be an interesting method of intra-procedural monitoring, especially combined with robotics, but currently MRI guidance is not a routine method of guidance in orthopedic surgery. In our experience, MRI directly after CT-guided ablation does not reliably show ablation extent.
Current perils and pitfalls of the computer assisted local ablation technique:
Requirement of CT based navigation means radiation exposure
Currently no means of modelling the local ablation in preplanning outside basic geometric matching
Navigated local ablation requires a high initial investment both in time and money
Currently no easy means of monitoring ablation progression and local temperature
MRI guidance currently expensive and not compatible with most RFA systems
Complications
RFA has proven to be a safe technique, although some complications have been reported. Mostly, they are due to heat effects in surrounding tissues, such as burning of the skin or cellulitis. However, if load bearing areas are ablated, there is an increased risk of spontaneous fracturing. The technique is contraindicated in patients with pacemakers and potentially other digital implants. By nature, MWA has the potential to lead to complications comparable to those caused by RFA, although they are not described in orthopedic literature, probably because of lack of reports on MWA. Even if cryoablation as adjuvant to intralesional surgery increases the risk of postoperative fracturing, this is not observed when modern cryoprobes are used in the palliative setting for bone metastases.
Results
Local ablation has quickly become the preferred method of treatment in osteoid osteoma. While outcome studies show a lower first procedure success rate, this is acceptable because of the much lower invasiveness associated with percutaneous treatment. In a pilot study of 20 patients the use of RFA has demonstrated the potential to completely eradicate cartilaginous tumour cells [15]. The authors describe that the positioning needs to be improved to increase treatment effectiveness. RFA has further been applied with success to chondroblastoma, osteoblastoma and other benign and intermediate grade tumors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








