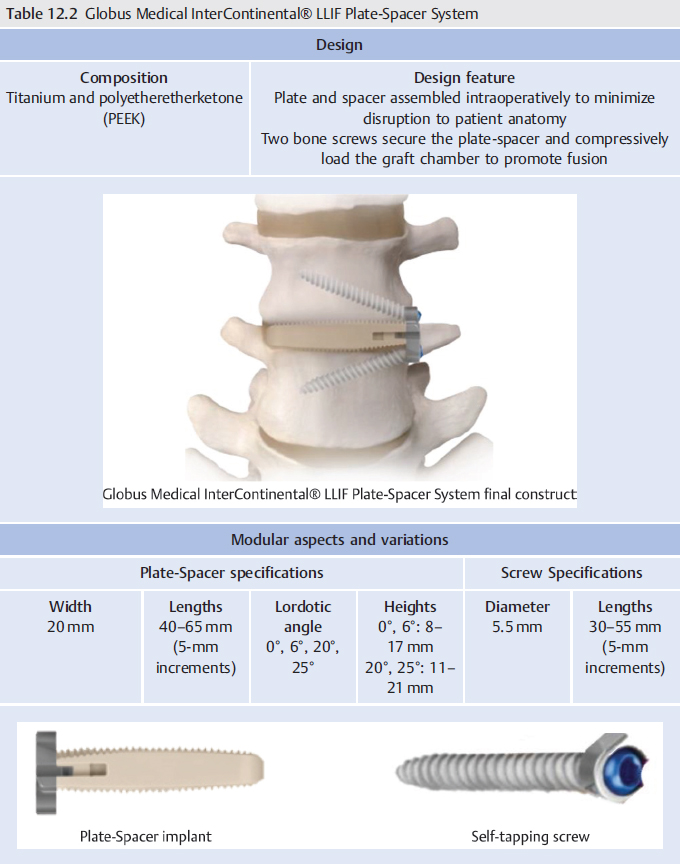
12 Lateral Fixation Systems
12.1 Introduction
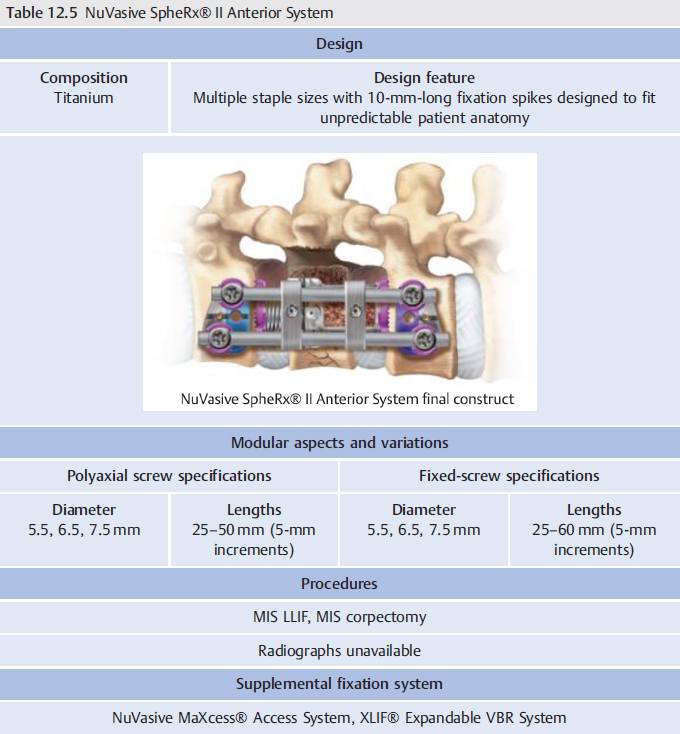
Vertebral plates are another type of instrumentation used for spinal fixation. Historically, lateral lumbar interbody fusions (LLIFs) were performed as stand-alone procedures.1 However, stand-alone interbody cages exhibited questionable stability due to limited resistance in vertebral motion.2 3 4 As such, supplemental fixation has been frequently utilized. However, posterior fixation in this setting requires patient repositioning and may increase the risk of complications and may increase morbidity.1 Lateral plates can be used in the setting of minimally invasive lateral approaches with the advantage of utilizing the same surgical approach as the interbody cage.1 This avoids the need for patient repositioning and reduces the risk of additional procedures. These instruments are often composed of a titanium plate with multiple screw slots for fixation to the vertebral body. Occasionally, the plate and screws are integrated with the interbody cage (Chapter 11 Lateral Interbody Cages) or VBR Device (Chapter 13 Vertebral Body Replacement Devices) in order to facilitate hardware placement.5 Surgical indications are presented in ▶ Table 12.1.
12.2 Outcomes
Screw–plate constructs have exhibited successful outcomes in reducing vertebral motion following interbody fusions.6 Lateral plates have been demonstrated to increase lumbar rigidity in flexion and extension.6,7 Lateral plates have also been noted to substantially reduce motion in lateral bending and axial rotation.8 Compared to bilateral pedicle screws (Chapter 3), screw–plate constructs have exhibited similar efficacy in terms of fixation and reducing vertebral motion. Furthermore, the utilization of lateral plates for fixation has been suggested to reduce patient morbidity.6,9 Previous studies have demonstrated shorter operative times, reduced blood loss, and decreased time under fluoroscopy.6 Plate instrumentation may also avoid many of the complications associated with posterior fixation, such as iatrogenic neurologic injury.6
Table 12.1 Surgical indications for vertebral plates
Indications |
• Lateral approach spinal fusion • Thoracolumbar procedures • Degenerative disk disease • Spondylolisthesis • Spinal fracture/dislocation |
12.3 Lateral Fixation Device Systems
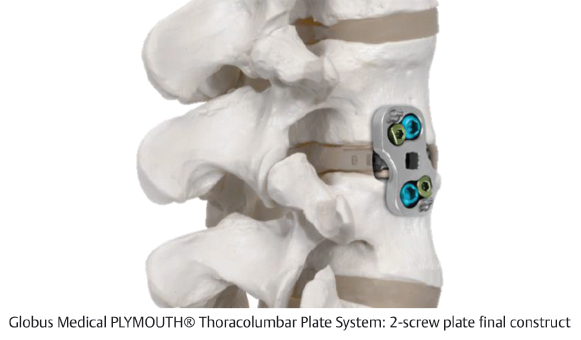
Table 12.3 Globus Medical PLYMOUTH® Thoracolumbar Plate System
Design | ||
Composition Titanium | Design feature 2- and 4-screw plate designs with simple locking set screw to allow visual confirmation | |
| ||
Modular aspects and variations | ||
Screw diameter 5.5 and 6.5 mm | Screw lengths 22–57 mm | Plate lengths 15–24 mm |
| ||
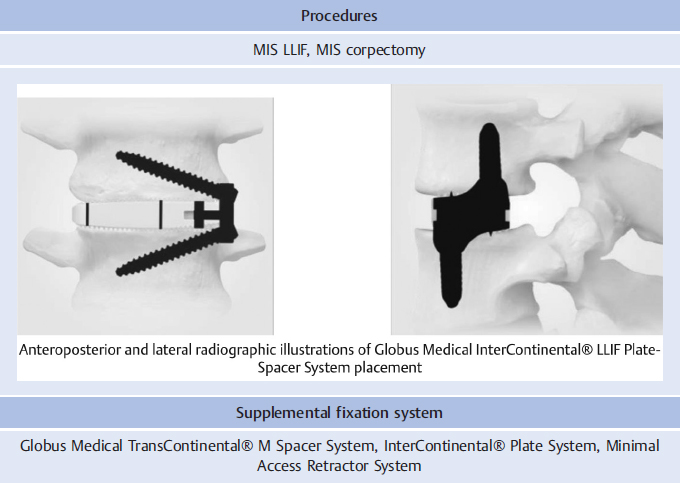
Procedures | ||
MIS LLIF, MIS corpectomy | ||
Radiographs unavailable | ||
Supplemental fixation system | ||
Globus Medical TransContinental® M Spacer System, CALIBER®-L Interbody Systems | ||
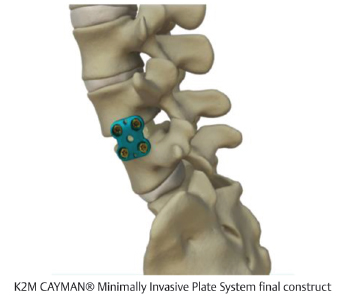
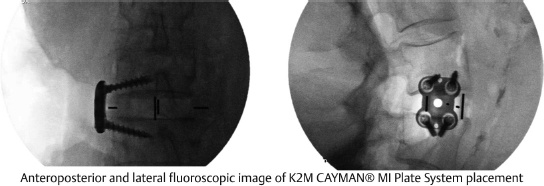
Table 12.4 K2 M CAYMAN® Minimally Invasive Plate System
Design | ||
Composition Titanium | Design feature TiFix locking technology enhances plate fixation and promotes stability | |
| ||
Modular aspects and variations | ||
Screw diameters 5 and 5.5 mm | Screw lengths 24–60 mm (4-mm increments) | Plate lengths 8–18 mm (2-mm increments) |
| ||
Procedures | ||
MIS LLIF, MIS corpectomy | ||
| ||
Supplemental fixation system | ||
K2 M RAVINE® Lateral Access System, ALEUTIAN® Lateral Interbody System | ||