Laboratory Diagnosis: Introduction
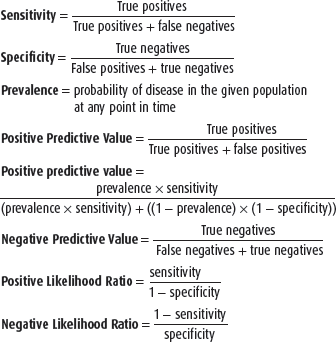
In general, laboratory tests are useful adjuncts in establishing a rheumatologic diagnosis but are not absolutely diagnostic of any specific disease. Two features of rheumatologic diseases contribute to the difficulties of interpreting laboratory tests. First, many rheumatic diseases are chronic systemic inflammatory diseases and, therefore, share many laboratory abnormalities with other such diseases, particularly chronic infections and malignancies. Second, the prevalence of certain rheumatologic diseases is low in most patient populations. Therefore, even if sensitivity and specificity of a test are high for a specific disease, the positive predictive value of the test may be low. Considering these statistical characteristics of laboratory tests can help the clinician interpret the data within the context of the clinical case.
Appropriate use of laboratory tests requires awareness of the rates and causes of false-positive and false-negative test results (see box, Defining Statistical Characteristics of Laboratory Tests). The sensitivity of a test demonstrates the ability of the test to detect a patient with disease and is measured by the proportion of people with disease who have a positive test result. The specificity of a test demonstrates the ability of the test to avoid detecting patients without disease and is measured by the proportion of people without disease who have a negative test result. The usefulness of a laboratory test is best reflected in the positive predictive value, which determines the proportion of patients with a positive test result who truly have the disease. The positive predictive value of a test depends on the prevalence of the disease in the population being examined (or the pretest probability of disease); thus, even if the sensitivity and specificity of a test are 99%, the positive predictive value of the test can be low if the prevalence of disease in the population is extremely low. The negative predictive value of a test determines how many patients with a negative test result truly do not have the disease. The negative predictive value also depends on the prevalence of the disease. The generally low prevalence of rheumatologic disease in the overall population means that many rheumatologic laboratory tests will only have a high positive predictive value when the tests are selected on the basis of clinical presentations that are highly suggestive of a rheumatologic disorder, which increases the pretest probability of disease.
The American College of Rheumatology (ACR) has published guidelines on some immunologic tests following review of the literature. Studies were evaluated for quality, and likelihood ratios were calculated through summary of the “best quality” studies.
The likelihood ratio (LR) is the likelihood that a given test result would be expected in a patient with the target disorder compared to the likelihood that that same result would be expected in a patient without the target disorder. The LR is used to assess the usefulness of a diagnostic test and to help the provider select appropriate tests for a given patient. The LR has the advantage of being independent of disease prevalence and indicates how much a given test result will raise or lower the odds of having disease relative to the pretest probability of the disease. An LR of 1 means that the posttest probability is exactly the same as the pretest probability.
Thus, an LR greater than 1 produces a posttest probability that is higher than the pretest probability. If the pretest probability lies between 30% and 70%, then positive test results with a very high LR (over 10) would rule in the disease.
An LR less than 1 produces a posttest probability that is lower than the pretest probability. A negative test result with a very low LR (below 0.1) virtually rules out the chance that the patient has the disease. A test is considered to be very useful if weighted average positive LR >5 or negative LR <0.2. A test is considered not useful if weighted average positive LR <2 or negative LR >0.5.
Autoantibodies
A variety of basic assays are used to detect autoantibodies. More than one type of assay may be available for any given autoantibody, and the particular test used may vary from institution to institution. In general, there has been a trend away from labor-intensive tests, such as agglutination assays and countercurrent immunoelectrophoresis, and toward assays amenable to automation, such as nephelometry, enzyme-linked immunoabsorbent assay (ELISA), and high throughput multiplex bead assays.
Indirect immunofluorescence assays identify autoantibodies reactive with antigens, in particular tissues or subcellular compartments (eg, nuclear antigens). Fixed tissue samples or cells are overlayed with patient sera and then washed. The presence of autoantibodies bound to the tissue sample is revealed by staining with a fluorescein-labeled antiserum against human immunoglobulin and observed by immunofluorescent microscopy. The antinuclear antibody (ANA) test has traditionally been done by this method.
Agglutination assays identify autoantibodies through the aggregation of particles, such as latex beads, coated with a defined autoantigen.
Immunodiffusion assays detect the formation of immune complexes in a semisolid support, such as an agar gel. Patient sera and antigen, placed in separate wells in the gel, diffuse toward one another and form a line of precipitation when insoluble immune complexes form. Placing the gel in an electrical field (countercurrent immunoelectrophoresis) increases the rate of diffusion and facilitates complex formation.
Nephelometry measures the interaction of antibodies and antigens in solution, detecting immune complex formation by monitoring changes in the scattering of an incident light.
ELISA uses an enzymatic readout to detect reactive antibodies. Sera to be tested for an autoantibody is incubated with the relevant autoantigen immobilized on a surface. After extensive washing, a detecting antibody (eg, an antiserum to human immunoglobulin) that has been conjugated to an enzyme is added. In the final step, substrate is added, and the product of the enzymatic reaction is measured. The amount of product reflects the quantity of detecting antibody bound to the autoantibody. There are several modifications of the basic ELISA, but all take advantage of the remarkable sensitivity imparted by the enzymatic readout.
High throughput multiplex bead assays use antigen-coated beads that are prelabeled with fluorescent label and mixed with serum and fluorescent-labeled secondary antibody. Multiplex bead assays can identify multiple autoantibodies and their specificities in single, high throughput assay. There is increasing use of this technology for ANA testing because (1) a small amount of serum is required, (2) the test is fully automated and more cost effective than measuring ANA by indirect immunofluorescence, and (3) the test provides simultaneous detection of ANA and identification of specific autoantibodies. However, a disadvantage is that only a limited group of antigens—not all nuclear antigens—are used, potentially lowering sensitivity. The test detects only autoantibodies to the selected set of nuclear antigens and does not detect all antibodies reactive with all nuclear proteins. Consequently, a negative multiplex test for ANA does not have the same negative predictive value for systemic lupus erythematosus (SLE) as does a negative indirect immunofluorescence test for ANA. Recent ACR guidelines state that the indirect immunofluorescence test for ANA remains the gold standard, and laboratories should inform providers of the method used to assess for ANA.
Rheumatoid Factor
Rheumatoid factor is an autoantibody directed against the Fc region of IgG. The most commonly used methods of detecting rheumatoid factor are latex fixation (using latex beads coated with human IgG) and nephelometry (using human IgG as the target antigen). Both assays primarily detect IgM rheumatoid factors. The results of latex fixation assays are reported as the greatest dilution that retains agglutination activity; in most laboratories, sera with titers of >1:40 are considered abnormal. Rheumatoid factor measured by nephelometry is quantified in international units, with ≥20 international units reported as abnormal in most laboratories. ELISAs for rheumatoid factor are also available but are not in wide use. ELISAs can measure IgG, IgA, and IgM rheumatoid factors.
Rheumatoid factor is present in 70–90% of patients with rheumatoid arthritis. Despite its name, rheumatoid factor is not specific for rheumatoid arthritis. Positive tests for rheumatoid factor occur in a wide range of autoimmune disorders, inflammatory diseases, and chronic infections (Table 3–1). Also, the prevalence of positive rheumatoid factor tests increases with age; as many as 25% of persons over the age of 65 may have a positive test result. In the absence of disease, the titer for rheumatoid factor is usually low (≤1:160). High titer for rheumatoid factor (≥1:640) almost always reflects an underlying disease.
|
Because of the large number of disorders associated with rheumatoid factor (see Table 3–1), the value of a positive test for rheumatoid factor depends on the pretest probability of the disease. In the proper clinical setting, a positive test provides strong support for the diagnosis of rheumatoid arthritis. However, it should be kept in mind that the combination of arthritis and a positive test for rheumatoid factor is not specific for rheumatoid arthritis and can be seen in patients with SLE; mixed connective tissue disease; systemic vasculitis; polymyositis; dermatomyositis; sarcoidosis; subacute bacterial endocarditis; and viral infections, particularly hepatitis C.
A negative test for rheumatoid factor should not be the only reason to rule out the possibility of rheumatoid arthritis. Between 10% and 30% of patients with long-standing disease are “seronegative.” At the time of presentation, however, the prevalence of a positive rheumatoid factor test is substantially lower (in the range of 50%). Therefore, the sensitivity of the test is lowest when the diagnosis is most likely to be in doubt. Although a positive rheumatoid factor test in a patient with clinical rheumatoid arthritis is associated with more severe, erosive disease and increased extra-articular manifestations of rheumatoid arthritis, there is no role for serial measurement of rheumatoid factor during the course of the disease for evaluation of disease activity.
Antibodies to Cyclic Citrullinated Peptides
Proteins that contain citrulline are the target of an autoantibody response that is highly specific for rheumatoid arthritis. Citrulline, a neutral amino acid, is not genetically encoded. Citrullinated proteins arise through a posttranslational modification in which arginine residues are converted enzymatically to citrulline. Currently, autoantibodies to citrullinated proteins are detected using ELISA with synthetic cyclic citrullinated peptides (CCP).
The presence of anti-CCP antibodies appears to be quite specific for rheumatoid arthritis. The second-generation ELISA tests for anti-CCP antibodies (anti-CCP2) have a specificity for rheumatoid arthritis as high as 97%. The sensitivities of anti-CCP tests are in the range of 70–80% for established rheumatoid arthritis and of 50% for early-onset rheumatoid arthritis. Thus, compared with rheumatoid factor, the currently available anti-CCP ELISA tests have superior sensitivity and comparable sensitivity for the diagnosis of rheumatoid arthritis. Recent studies suggest that a rheumatoid factor test does not add to the ability of an anti-CCP2 test to predict joint damage in patients with rheumatoid arthritis and does not add to ability of anti-CCP2 to predict progression from undifferentiated arthritis to rheumatoid arthritis.
The likelihood of rheumatoid arthritis developing in individuals with positive test results for both rheumatoid factor and CCP2 is nearly 100%. Most patients with rheumatoid arthritis are positive for both anti-CCP antibodies and rheumatoid factor, but some have only one of these autoantibodies, and others have neither. A meta-analysis of studies of anti-CCP in rheumatoid arthritis demonstrated a pooled sensitivity of 67%, specificity of 95%, a positive LR of 12.46, and a negative LR of 0.36. The risk of radiographic progression was shown to be greater with positive anti-CCP than positive rheumatoid factor in patients with rheumatoid arthritis.
The presence of anti-CCP antibodies provides strong support for the diagnosis of rheumatoid arthritis. Moreover, in patients with early onset, undifferentiated, inflammatory arthritis, the presence of anti-CCP antibodies is a strong predictor of progression to rheumatoid arthritis and for the development of joint erosions. A positive anti-CCP antibody test or a positive rheumatoid factor test is part of the 2010 ACR diagnostic criteria for rheumatoid arthritis. A negative test for anti-CCP antibodies does not exclude the possibility of rheumatoid arthritis, particularly at the time of initial presentation when approximately 50% of patients lack detectable anti-CCP antibodies.
The specificity of the anti-CCP ELISA tests suggests that this test will prove useful when determinations of rheumatoid factor are not. For example, initial studies indicate that anti-CCP antibodies are not associated with chronic hepatitis C infection. In contrast to rheumatoid factor, therefore, testing for anti-CCP antibodies may help distinguish concomitant rheumatoid arthritis from viral arthritis in patients infected with hepatitis C.
Antinuclear Antibodies
Antinuclear antibodies (ANAs) are autoantibodies directed against histones, double-stranded and single-stranded DNA, ribonucleoprotein (RNP) complexes, and other nuclear components. ANAs are measured using either indirect immunofluorescence assays, ELISA, or high throughput multiplex bead assays.
Current indirect immunofluorescence assays for ANA use HEp-2 cells, a human epithelial cell line, as the source of nuclei and are more sensitive than older tests that used rodent liver and kidney. These assays for ANA report the titer of the ANA and the pattern of nuclear staining and are considered the gold standard for ANA testing. In most laboratories, ANA with titers ≥1:40 are considered positive. The staining patterns are diffuse or homogeneous (due to antibodies to histone), rim (an uncommon pattern due to antibodies to nuclear envelope proteins and to double-stranded [ds] DNA), speckled (due to antibodies to Sm, RNP, Ro/SS-A, La/SS-B, and other antigens), nucleolar (see the section, Antibodies to Nucleolar Antigens, below), and centromeric. In general, there is a poor correlation between the pattern of the ANA and the identity of the underlying disease. An exception is the centromeric pattern, which has considerable specificity for limited scleroderma (see the section, Anticentromere Antibodies
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree