Fig. 1
Double-collagenase digestion procedure for VSMC isolation from aorta
3.2 Mouse Aorta Dissection
1.
Select mice from which aortas will be dissected. Usually 5–10 mice are required for one VSMC isolation procedure (see Note 4 ). The same protocol can be used for rat aortas.
3.
Spray the mouse with 70 % of ethanol and make an initial cut in the lower part of the abdomen.
4.
Cut the skin from both sides to visualize the organs and the rib cage.
5.
Open the chest cavity by cutting the ribs from both sides to access the heart.
6.
Remove or move organs (liver, spleen, intestine, etc.) to reach the aorta, which is visible as a white tube running along the spine.
7.
With scissors, cut the abdominal aorta below the renal arteries.
8.
With a syringe, inject 5 mL of PBS into the left ventricle in order to flush blood out of the aortic lumen.
9.
Carefully dissect the aorta away from the vertebral column using small surgical scissors (straight or curved) and forceps (see Note 6 ). Start above the abdominal part of the aorta where the diaphragm is attached and work towards the heart.
10.
Separate the aorta from the heart; make a cut before the aortic arch to obtain only the thoracic part (see Note 7 ). Put the isolated aorta in a culture dish containing PBS (see Fig. 2a).
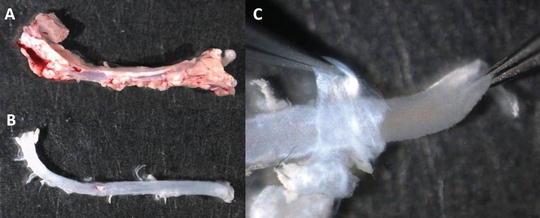

Fig. 2
Cleaning mouse aorta. (a) Aorta freshly dissected from a mouse. (b) Aorta after removal of the surrounding tissue. (c) Removal of the adventitia (held with left tweezers) from the medial layer (held with right tweezers)
11.
Follow the same procedure (steps 2–7) for all mice. Store all the dissected aortas in PBS until further use (next Subheading 3.3).
3.3 Vascular Smooth Muscle Cell Isolation and Culture
1.
2.
Transfer clean aortas into a culture dish containing fresh PBS.
3.
4.
Put one aorta in a culture dish with fresh PBS.
5.
6.
Repeat steps 4 and 5 for all the aortas.
7.
Place the medial aortas in a sterile culture dish containing sterile PBS using a sterile scalpel blade. From this step work in STERILE conditions!
9.
10.
At the end of the digestion period, remove the stir bar with sterile forceps. Centrifuge the cell/collagenase mixture for 5 min at 300 × g. Remove the supernatant and resuspend the cell pellet in 50 mL of sMEM.
11.
Centrifuge the suspended cells for 5 min at 300 × g. Remove the supernatant and resuspend the cell pellet in an appropriate volume of sMEM supplemented with 10 % FBS (see Table 1).
Table 1
Growth area and medium volume for selected cell culture vessels
Approx. growth area (cm2) | Recommended medium volume (mL) | |
|---|---|---|
Multiple-well plates | ||
6 well | 9.5 | 1.9 |
12 well | 3.8 | 0.8 |
24 well | 1.9 | 0.4 |
48 well | 0.95 | 0.2 |
Dishes | ||
35 mm | 9 | 1.8 |
60 mm | 21 | 4.2 |
100 mm | 55 | 11 |
Flasks | ||
25 cm2 | 25 | 5 |
75 cm2 | 75 | 15 |
12.
13.
After 2 days, remove the sMEM (with debris) and add fresh sMEM supplemented with 10 % FBS. Under the microscope, confirm the presence of adherent cells (see Fig. 3a).
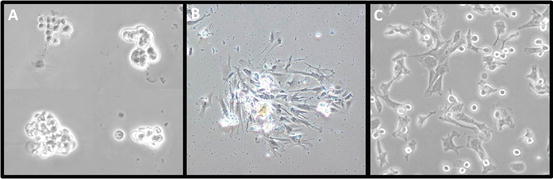

Fig. 3
Primary culture of vascular smooth muscle cells (VSMCs). VSMCs were incubated at 37 °C in 5 % CO2 with MEM supplemented with 1 mM l-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10 % FBS. (a) Groups of VSMCs after 2 days of culture. (b) A group of adherent VSMCs after 1 week of culture. (c) VSMCs replated after the first trypsinization passage
14.
Change the medium every 2 days (use sMEM supplemented with 10 % FBS), each time observing cells under the microscope to monitor cell growth.
15.




When numerous cell colonies are formed (see Fig. 3b), wash cells twice with PBS and add 0.5 mL of 1× trypsin per well (see Note 15 ); incubate for ~5 min at 37 °C in 5 % CO2 until cells detach.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







