CHAPTER 19
Intrathecal Baclofen for Spasticity
Gerard E. Francisco and Michael Saulino
Intrathecal baclofen (ITB) therapy is a potent method for management of spastic hypertonia and related features of the upper motor neuron syndrome (UMNS). ITB infusion exerts its therapeutic effect by delivering baclofen directly into the cerebrospinal fluid (CSF) with rapid distribution to target neurons in the spinal cord. Intrathecal administration of baclofen is typically effected through use of an externally programmable, surgically implanted pump, delivering drug at precise flow rates via a catheter placed in the spinal canal. Studies demonstrate evidence of effectiveness of the ITB infusion system in reducing hypertonia in patients with cerebral palsy, spinal cord injury, multiple sclerosis (MS), and acquired brain injury due to stroke, trauma, or hypoxia. Although most of the information presented herein is derived from studies of adults treated with ITB for severe spasticity, discussion also specifically deals with clinical experience in pediatric and ambulatory patient populations. This chapter reviews important aspects of ITB therapy in the treatment of patients with multifocal, dysfunctional spasticity.
HISTORY OF ITB THERAPY
Intrathecal administration facilitates delivery of neurologically active drugs to target receptors in the central nervous system (CNS) indirectly via diffusion through the CSF. Although this technique has recently attained relatively widespread use, its origin can be traced back more than a century. In 1898, Bier reported the first therapeutic application of intrathecal therapy, performing spinal anesthesia through the use of intrathecal cocaine (1). In 1901, a Romanian physician, Racoviceanu-Pitesti, described the use of opiates for intrathecal anesthesia. Spinal drug administrations subsequently became one of the foundations of modern anesthesiology (2).
Intrathecal drug administration was initially limited to short-term use, however, and nearly eight decades would pass before physicians attempted to implement this therapy for chronic conditions. Onofrio et al described the beneficial effects of intrathecal morphine administration for treatment of chronic pain associated with cancer (3). In 1984, Penn and Kroin described the successful use of continuous intrathecal baclofen (CITB) infusion for reducing spasticity of spinal origin (MS or spinal cord injury) (4). Several clinical trials then preceded U.S. Food and Drug Administration (FDA) approval of ITB therapy for spasticity of spinal origin in 1992 (5). Subsequent investigations expanded the role of this therapy to spasticity of cerebral origin (stroke, cerebral palsy, and brain injury), with FDA approval in 1996 (6).
ITB INFUSION—NEUROPHYSIOLOGIC MECHANISM OF ACTION
As introduced previously, the rationale underlying intrathecal infusion therapy is to facilitate delivery of drugs to their respective sites of action within the CNS. This also reduces certain types of adverse effects because systemic drug exposure is reduced or bypassed entirely. Any drug that exerts a useful pharmacologic effect within the CNS and is capable of being maintained in a stable sterile solution can be considered a potential agent for intrathecal delivery. Although several drugs are commonly used for chronic intrathecal administration, currently only three have FDA approval for long-term use: baclofen (for spasticity), morphine (for pain), and ziconotide (for pain).
ITB’s most prominent neurophysiological effect involves the reduction of spinal reflex responses, both monosynaptic (H-reflex) and polysynaptic (flexion withdrawal reflex), in a dose-dependent manner (5,7–9). This is consistent with clinical observations of rapid disappearance of tendon taps and decrease in frequency and severity of muscle spasms with ITB. Biomechanical and neurophysiological studies also provide evidence of decreased resistance to imposed passive stretch, accompanied by a decrease in threshold and magnitude of electromyographic (EMG) response (10). The association of ITB with clinical reduction in muscle hypertonia, although present, is not as straightforward as its effect on reflex activity. Furthermore, convincing data remain elusive regarding the degree to which ITB can improve voluntary motor control. A few anecdotal cases of reduced agonist–antagonist EMG cocontraction have been reported, documenting an improved pattern of voluntary muscle activation during simple motor tasks (5,7,9,11,12), some of which coinciding with better functional outcomes (11).
At the neuronal level, baclofen acts as a potent gamma-aminobutyric acid (GABA)-B receptor agonist. GABA-B receptors are extensively distributed in the spinal cord, making this spinal neuronal network a prime target for this drug’s antispastic effects. Baclofen administered directly to the subarachnoid space has enhanced access to GABA receptors compared to oral administration, and thus allows greater reflex inhibition and hypertonia reduction (13–15). The exact mechanism of baclofen action, however, remains elusive. Baclofen could interfere with signal transmission along various afferent pathways and with neurotransmitter release (presynaptic), or alter motor neuron physical properties and their excitability (postsynaptic). Although presynaptic mechanisms have long been favored, postsynaptic baclofen effects on motor neurons and interneurons have more recently been reported in experimental animals (16–18). These offer a plausible explanation for some neurophysiologic studies in humans, which favor post- over presynaptic site of baclofen action. For example, Azouvi et al (8) reported no discernable effect of baclofen on vibratory inhibition or heteronymous facilitation of the H-reflex, thereby refuting the contribution of presynaptic mechanisms to inhibition of monosynaptic reflexes. Additional support for the postsynaptic action can be found in decreased F-wave frequency after ITB bolus (10) or CITB administration (19). At clinically achievable ITB doses, the suppressive effect of ITB on mono- and polysynaptic reflex pathways seems to be presynaptic, most probably by interfering with neurotransmitter release, with postsynaptic effects possible at higher doses (20).
COMPONENTS OF ITB THERAPY
Intrathecal delivery systems are comprised of a few key components. These include an accessible drug reservoir, a method of propelling the drug out of the pump and a catheter that connects the drug reservoir to the CSF (Figure 19.1). Although constant flow systems are available (21), programmable systems are overwhelmingly preferred due to their adjustability for individual patient need and response (22). Additional components are needed for programmable systems, including an external programming device and a communication method between the programmer and the implanted pump. The propulsion technique for variable controlled pumps is typically electronic, necessitating an energy source to drive the system. In contrast, constant flow pumps can utilize a pneumatic propulsion technique. Future components may include automated troubleshooting of the system, self-directed patient programming, and integrated sensors to detect patient position and functional activity level.
FIGURE 19.1 Schematic diagram of intrathecal delivery system.
ITB infusion systems afford potent relief of spastic hypertonia, although some clinical presentations are better suited for this therapy. Graham and colleagues used a grid illustration to compare characteristics of various therapies for spasticity, contrasting reversible versus irreversible options and focal versus global effects (Figure 19.2). In this model, ITB was considered reversible (neural structures are not surgically altered, and rate of dose administration is adjustable) and global (CNS effects of ITB distribution are typically observed in all extremities and the trunk). Thus, patients with global or multifocal spasticity who may benefit from an adjustable (vs permanent) clinical effect are generally considered better candidates. Furthermore, ITB can be combined with other modalities for synergistic therapeutic effect, including rehabilitative therapies, oral pharmacotherapy, neurolytic procedures, and muscle/tendon lengthening procedures. Physical techniques such as stretching, strengthening, bracing, and gait retraining are essential for attaining maximal functional benefit that may follow tone reduction. Patients might continue to use oral spasticity agents for a variety of reasons including ongoing ITB titration, “breakthrough” spasms, an irregular spasticity pattern, disease progression, or residual upper extremity tone. Combining ITB therapy and neurolytic procedures is appropriate for patients manifesting both focal dystonic features and global hypertonicity, or residual upper extremity hypertonia (24). The indications for combining neuro-orthopedic procedures and ITB therapy include correction of fixed deformities in the presence of ongoing spastic hypertonia. Concomitant use of orthopedic surgery and ITB in children with CP may reduce the need for subsequent orthopedic surgery (25).
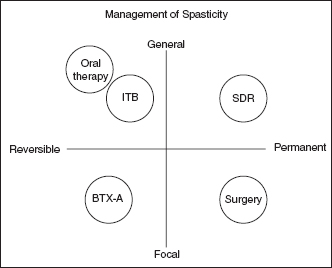
FIGURE 19.2 Characterization of various spasticity treatment modalities.
Source: From Ref. (23). Graham HK, Aoki KR, Autti-Rämö I, et al. Recommendations for the use of botulinum toxin type A in the management of cerebral palsy. Gait Posture. 2000;11(1):67–79.
Patient Selection and the Role of Preimplant Trials
In terms of higher cost, potential benefit, and complication risk, few current spasticity treatments compare with ITB infusion therapy. This form of therapy may be optimal for some patients, and yet unsuitable for others with comparable degrees of moderate or severe spasticity. Patient selection and education are important to achieving optimal outcomes. Appropriate candidates need to be counseled regarding that proceeding with this form of therapy represents a long-term commitment.
In general, patients can be considered candidates for ITB therapy when:
• spasticity is poorly controlled despite maximal therapy with other modalities
• spasticity is poorly controlled because of limited patient tolerance of other modalities
• adjustable spasticity reduction afforded by a programmable variable flow pump would be advantageous
Patients should be clinically stable, understand the risks and benefits of ITB therapy, be able to return to clinic for titration and refills, and have demonstrated a positive response to a test dose of ITB. In severe cases, this therapy can be considered early in the postinjury recovery period (eg, <12 months [26]). ITB therapy generally reduces lower limb hypertonia to a greater extent than in upper extremities. More cephalad catheter tip placement, however, can potentially improve upper limb response (27). Other advantages of ITB therapy include higher potency with potentially less adverse effects compared to oral baclofen, the ability to have a global effect on all affected limbs, and the possibility of later adjustment with changing patient need or progressive disease. Disadvantages include surgical risks (bleeding, infection, and damage of neural structures), the potential for serious adverse effects including overdose and withdrawal, and the requirement for ongoing follow-up with health care professionals for dosing adjustments and pump refills. Ventricular shunting for hydrocephalus is not a contraindication to ITB therapy, but practitioners should be aware of potential interactions between the devices on CSF flow (28). ITB can also be used in patients with seizures with the understanding that this therapy has been occasionally associated with an increased risk of seizures (29,30).
For the patient who chooses this form of therapy, the preimplant trial is the first of four phases of ITB treatment: (a) trial, (b) surgery, (c) titration, and (d) maintenance. The preimplant trial involves administration of a test dose of ITB to assess the patient’s response to this agent. Typically, a lumbar puncture is performed, and a bolus of a baclofen solution is injected into the CSF. Fifty micrograms of baclofen is the most commonly used initial screening dose (31,32). The onset of clinical effects from a screening bolus occurs within 1 to 3 hours postinjection and peak effects are typically observed 4 to 6 hours postinjection. The effects of the screening bolus are always temporary with the effects routinely lasting 6 to 8 hours (10,33). Prolonged effects of single test bolus have been reported (34). Screening boluses can be repeated if the initial injection is unsuccessful. “Positive” responses are reported in 80% to 90% of bolus trials (31). Generally, antibiotic prophylaxis is not needed for a bolus trial (35). For patients on antiplatelet or anticoagulant therapy, recommendations from the American Society of Regional Anesthesia are followed (36). Fluoroscopic guidance can assist needle localization into the intrathecal space because anatomic landmarks for lumbar puncture can be variable.
An alternative method for conducting trials involves placement of a temporary intrathecal catheter and monitoring patient response to a short-term continuous infusion of baclofen (37). This technique is more commonly utilized for evaluating chronic pain patients for intrathecal opiate therapy. The specifics of catheter placement are described later in this chapter. The advantages of catheter ITB trials include (a) avoidance of sequential lumbar punctures, (b) presumably improved approximation of chronic postimplant intrathecal infusion response when compared to single bolus injections, (c) the ability to control catheter tip placement for evaluation of upper extremity effects, and (d) the ability to adjust infusion rate while assessing favorable (and unfavorable) effects of ITB administration. The disadvantages of catheter trials include increased technical difficulty, increased need for observation, and increased risk of meningitis and structural damage. Fluoroscopic guidance is generally considered mandatory for catheter placement. Although antibiotic prophylaxis is usually not needed for bolus trials, it is unclear whether antibiotic prophylaxis is needed for short duration intrathecal catheter trials. Factors to consider include the duration of the trial, patient immunocompetency, and potential chronic bacterial colonization. Evidence suggests that trial duration is a key risk factor for development of infectious complications. Thus, the trial should last only as long as required to indicate a potential benefit of chronic ITB therapy (35,38). There is no consensus regarding the optimal method of anesthesia used for catheter placement. Local anesthesia potentially lowers the risk of inadvertent damage to neural structures. However, if excessive patient movement or severe anxiety is anticipated, deep sedation or general anesthesia may be warranted (39). There is little evidence to suggest that catheter trials provide better long-term outcomes compared to bolus trials when used as predictors of postimplant response. Prospective data in the pain management literature suggest no difference in outcomes from either intrathecal trial method (40).
Definitions of “success” for screening trials vary. A more liberal description of a successful trial might be any improvement in spasticity that suggests future benefit from chronic long-term infusion. Subjective patient reports can be used to assess spontaneous spasm frequency and intensity (41). The most commonly cited criterion for a successful ITB trial is a 2-point reduction on the Modified Ashworth Scale (MAS) (31). Patients may also demonstrate improvement in joint range of motion (ROM), both actively and passively. ITB trials can potentially differentiate ROM deficits due to severe spasticity, which are potentially reversible without surgery, from fixed contractures.
Although these evaluation techniques are often useful for patients with hypertonia in resting positions, these assessments may be inadequate for prediction of ITB effect during active functional tasks. In these patients, excessive tone reduction may impede performance of activities such as transfers and walking. Observation of ambulation, transfers, posture, and wheelchair propulsion during the trial is thus warranted. Adjunctive objective evaluation techniques may be helpful, and include neurophysiologic assessment (10) and instrumented gait analysis (33). There is an inconsistent correlation between subjective report and objective measures of spasticity (42). During a screening trial, some individuals may experience excessive spasticity reduction during the peak effect of the ITB bolus. This occurrence is not a contraindication for pump implantation because the chronic infusion system has the ability to modulate dose and subsequent desired effect. If excessive or prolonged hypotonia is observed during a screening trial, a repeat trial at a lower dose or continuous trial may be warranted. Particular care should be given to patients who demonstrate an improvement on “passive” measures of spasticity (thus qualifying for long-term infusion on the basis of trial “success”), yet demonstrate functional worsening during the trial. Postimplant rehabilitation in this subset of patients is particularly important.
Adverse effects can occur during the test phase. Spinal headache or post-lumbar puncture syndrome is a complication of an injection-related dural leak and is not a direct medication effect. Spinal headaches occur in up to 30% of patients undergoing a lumbar pucture and can vary in severity from mild to incapacitating. Headache typically worsens when the patient sits or stands up and decreases in the supine position. These headaches typically begin within 2 days, but may be delayed as long as 2 weeks (43). Spinal headaches can be accompanied by dizziness, neck or arm pain, cranial nerve palsies, tinnitus, nausea, and distorted vision (43). Spinal headaches are more common in younger women with a low body mass index and in people who have a headache history in general. The risk of spinal headaches increases with use of larger needles. The headache resolves spontaneously in the majority of patients. Supportive measures include bedrest, caffeine, and abdominal binders. Epidural blood patch is reserved for recalcitrant cases (43). Other procedure-related complications include bacterial and aseptic meningitis. Adverse effects that are more likely related to a pharmacologic effect include nausea/vomiting, urinary retention, hypotension, seizures, drowsiness/sedation, respiratory depression, and coma. Nausea/vomiting and drowsiness/sedation are the most common adverse effects observed during ITB trial with reported incidences of 2% to 3% (31).
Although the procedural component of the trial should take place in a setting where injection sterility is ensured, a number of settings are suitable for monitoring the effects of the trial. Examples include outpatient clinics, ambulatory surgical centers, inpatient hospitals, and inpatient rehabilitation facilities. Furthermore, it is helpful to use practice protocols or pathways to facilitate consistent assessment of key trial response indicators and reduce the risk of complications. As described previously, sequential evaluations of tone, range of motion, and strength are required. For patients who utilize spasticity to assist with functional mobility, similar sequential evaluations of posture, transfers, and gait should be undertaken. Protocols for management of adverse events should be in place including spinal headache, bowel and bladder changes, seizures, and respiratory depression. Because the effects of ITB trials are occasionally prolonged, the practice setting should have the capacity for extended observation. Many experienced practitioners of ITB therapy believe that inpatient rehabilitation facilities are an optimal site for trials because these locations offer the best ability to assess functional changes and potentially manage adverse effects.
Some centers proceed directly to pump implantation without a screening trial. For stroke patients, two justifications have been proposed—(a) the increased risk of spinal hemorrhage while patients are in anticoagulation or antiplalelet therapy and (b) the risk of recurrent stroke if these agents are discontinued. This method reduces the ability to differentiate fixed contracture from spasticity prior to implantation. Although patients may still benefit, it should only be undertaken after a full discussion with the patient and caregivers regarding the risks and benefits of a “no trial” approach.
Implantation
Once a positive trial response has been observed, a patient may proceed to pump implantation. Patients should be clinically stable prior to surgery to minimize perioperative complications. Preoperative antibiotics are typically utilized. Patients on chronic anticoagulation will need to discontinue medications in the days preceding the procedure (36). The risks of the permanent pump implantation and infusion are same as the screening trial with the additional risks of drug overdose, drug withdrawal, and device complications.
Various options for pump and catheter placement should be considered before the procedure. The vast majority of intrathecal pumps in commercial use in the United States are manufactured by Medtronic. Johnson and Johnson had FDA approval for programmable pump (Medstream), but this device has been removed from clinical use. Flowonix has a programmable pump for chronic pain (Prometra). This device is not approved for baclofen at present. The MRI compatibility of this device is different from the Synchromed system. Currently, it is recommended that drug be removed from the pump reservoir prior to the MRI scan. One potential advantage of this system is the capability to deliver very low flow rates including a stopped pump mode without damaging the delivery system. A few other companies are in early-stage development of programmable systems. The size of the implanted pump should be determined based on the patient’s body habitus and anticipated intrathecal dosing. Smaller and thinner individuals might prefer a smaller pump size, either for esthetic reasons or to prevent erosion of the pump through the skin and subcutaneous tissue. Similarly, the pump can be placed under abdominal fascia for similar reasons (44). Patients who are anticipated to require high ITB doses or who reside a great distance from the follow-up clinic will benefit from larger pumps with larger drug reservoirs and longer refill intervals. The tip of the intrathecal catheter is routinely placed in the mid-lower thoracic region, particularly if reduction of lower extremity spasticity is the primary concern. More rostral tip placement can be attempted to improve upper extremity hypertonicity (27,45).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






