INTRODUCTION
Chronic pain is one of the most common medical problems encountered by physicians. It has been described as an unpleasant sensation that persists for at least 4 months, and often continues for an indefinite period of time. In the United States alone, more than 90 million people suffer from a variety of chronic pain syndromes. Chronic pain often disrupts family life, reduces function, and is a financial strain. Chronic pain is more common and causes more disability than cancer and heart disease combined. The drain on the U.S. economy produced by chronic pain is enormous, amounting to more than 100 billion dollars a year in medical expenses, lost work productivity, and insurance costs.
Use of interventional pain management procedures in the treatment of chronic benign and malignant pain can offer significant pain relief, improving function and reducing medical costs for patients, and provides a better quality of life and death for those with terminal disease. This chapter reviews indications, contraindications, complications, efficacy, and techniques for the most common interventional procedures used in the treatment of chronic pain.
SYMPATHETIC BLOCKS
The sympathetic nervous system works in conjunction with the parasympathetic nervous system to provide homeostasis for many bodily functions. Sympathetic nerves provide innervation to a variety of structures, including the skin, blood vessels, and internal organs. The sympathetic nervous system can lead to ongoing chronic neuropathic pain involving the extremities, face, abdomen, and pelvis. Sympathetic nerve blocks are often used to treat a spectrum of neuropathic disorders from sympathetically maintained pain to complex regional pain syndromes. The pain can be mild and self-limited, as in a sunburn, or severe and chronic, as in trigeminal neuralgia, complex regional pain syndrome, and diabetic peripheral neuropathy. Sympathetic nerve blocks are used diagnostically to identify a neuropathic disorder (eg, complex regional pain syndrome) and therapeutically in the treatment of neuropathic conditions that have not responded to more conservative care.
Cervical sympathetic injections are performed to treat neuropathic facial pain, such as trigeminal neuralgia, headaches (cluster, migraine), and atypical facial pain. These nerve blocks can also be used in the treatment of facial pain related to different types of cancer involving the head and neck. They are also used for neuropathic upper extremity pain, as seen with sympathetically maintained pain, radiculopathy, and complex regional pain syndromes. An increased temperature recorded in the involved extremity reflects a successful injection resulting from blockade of the sympathetic nerves. In regard to facial pain, the observation of a Horner’s syndrome postinjection, consisting of ptosis, enophthalmos, miosis, and anhidrosis, confirms an effective blockade.
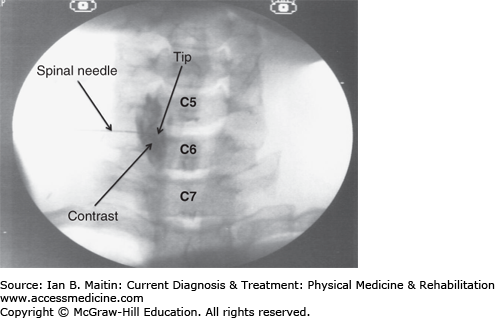
The cervical sympathetic ganglion, also known as the stellate ganglion, is located anterolateral to the C7 vertebral body and consists of a union between the inferior cervical and first thoracic sympathetic ganglia. The injection procedure is performed with the patient in the supine position. The anterolateral portion of the neck on the involved side is prepared and draped in a sterile fashion. The anterolateral aspect of the C7 vertebral body is located using fluoroscopic visualization. This is achieved by rotating the C-arm to the ipsilateral side and positioning the image intensifier such that a transforaminal view is obtained with sharp endplates.
The skin overlying the C7 uncinate process is anesthetized with a local anesthetic. A 25- or 22-gauge, 3½-inch spinal needle is then inserted through the skin and advanced under fluoroscopic visualization until the needle tip makes contact at the base of the uncinate process. Next, the needle stylet is removed and tubing is attached to the needle hub. The other end of the tubing is attached to a syringe containing nonionic radiopaque contrast. The syringe is then aspirated to ascertain if the needle tip has entered any vascular structure.
The contrast is injected after negative aspiration under continuous fluoroscopy to further evaluate any violation of vascular structures. The contrast pattern of the properly placed needle tip should outline the surrounding muscular and osseous structures without any vascular uptake (Figure 21–1). The anteroposterior and lateral fluoroscopic views are used to confirm needle placement and contrast pattern. Lastly, the contrast syringe is exchanged for the syringe containing the injectate, which typically consists of a long-acting local anesthetic, such as bupivacaine.
Side effects and complications associated with cervical sympathetic blocks can include infection, hematoma with subsequent airway compromise, injury to exiting nerve roots, vascular damage, and trauma to the spinal cord.
An evidence-based systematic review of sympathetic injections published by Day in 2008 shows a paucity of literature regarding the utility and outcome of all types of sympathetic injections. Only one prospective, double-blind, placebo-controlled study had been reported; the remainder of the included literature consisted of case reports, case series, and one retrospective study. Day concluded that the evidence was very low quality.
Thoracic sympathetic injections are typically performed when neuropathic upper extremity pain does not respond to cervical sympathetic injections. In approximately 20% of people the thoracic sympathetic fibers that normally contribute to the stellate ganglion bypass it and merge with the brachial plexus. In those situations, blocking the T2 and T3 sympathetic ganglia, which are located anterolateral to their respective T2 and T3 vertebral bodies, provides relief of the neuropathic upper extremity pain.
There are two techniques for blocking the T2 and T3 sympathetic ganglion. The anterior (paratracheal) approach involves placing the spinal needle tip at the ventral surface of the C7 transverse process. Subsequent injection of local anesthetic then tracks in a superior and inferior direction blocking the T2 and T3 sympathetic ganglia. The risks associated with this technique include pneumothorax due to the proximity of the lung apex, and injury to the vertebral artery, which is located anterior to the C7 process. The posterior approach, which is described below, has a significantly greater success rate and is much safer than the anterior approach.
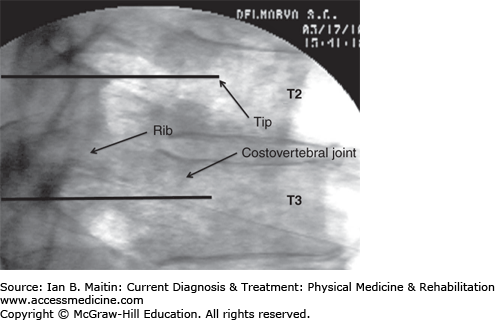
The patient is positioned prone when performing a T2 sympathetic ganglion injection. The neck and upper thoracic area are prepared and draped in a sterile manner on the symptomatic side. The C-arm is rotated obliquely approximately 10 degrees toward the ipsilateral side.
The skin is anesthetized with a local anesthetic and a 22- or 25-gauge, 3½-inch spinal needle is passed percutaneously and advanced under fluoroscopic guidance until the needle tip makes contact with the posterior lateral margin of the T2 vertebral body. The needle tip is then advanced carefully underneath the rib and further advanced in the lateral plane until the tip is located anterior to the costovertebral joint (Figure 21–2).
Side effects and complications associated with thoracic sympathetic injections include nerve injury, pneumothorax, and intravascular injection.
The literature is lacking in regard to thoracic sympathetic injections.
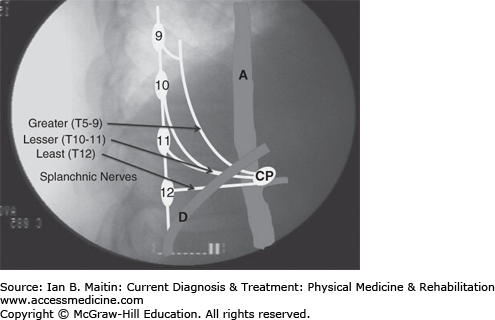
Celiac plexus and splanchnic sympathetic blocks have been used predominately in the treatment of cancer-related abdominal pain but can also be used in patients with chronic nonmalignant abdominal pain after the failure of conservative treatment. The celiac plexus is located at the L1 level anterior to the aorta (Figure 21–3). The preganglionic fibers from T5 to T12 form the greater (T5–9), lesser (T10–11), and least splanchnic (T12) nerves, which travel along the lateral and anterolateral aspects of the T9–12 vertebral bodies. All three splanchnic nerves traverse the T12 vertebral body before passing through the diaphragm to synapse at the celiac plexus (see Figure 21–3).
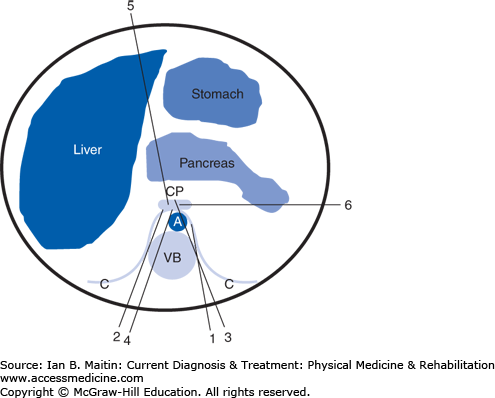
Multiple techniques for performing celiac plexus blocks have been described (Figure 21–4), including bilateral or unilateral paravertebral retrocrural injection (splanchnic sympathetic blocks), bilateral or unilateral posterior paravertebral antecrural injection, posterior transaortic injection, transintervertebral disc injection, anterior abdominal injection, and lateral abdominal injection. Most of these techniques can be performed using either fluoroscopic or computed tomographic (CT) guidance; the exceptions are the anterior and lateral abdominal approaches, which typically use CT guidance or transcutaneous ultrasound, in the case of the anterior abdominal approach.
Figure 21–4
Celiac plexus sympathetic injection techniques. (1) Unilateral posterior paravertebral retrocrural splanchnic injection. (2) Unilateral posterior paravertebral antecrural plexus injection. (3) Posterior transaortic plexus injection. (4) Transintervertebral disc plexus injection. (5) Anterior single needle abdominal plexus injection. (6) Lateral abdominal plexus injection. CP, celiac plexus; A, aorta; VB, vertebral body; C, diaphragm crura.
Another unique approach to performing a celiac plexus injection involves the use of an upper gastrointestinal endoscope with an ultrasound curvilinear transducer (echoendoscope). The endoscope is positioned at the distal end of the esophagus and the aorta is identified longitudinally by ultrasound. The celiac artery is identified at the point where it branches off from the aorta as the endoscope is advanced distally toward the proximal stomach. A 22-gauge aspiration needle is inserted through and attached to the biopsy port and subsequently visualized by the ultrasound transducer anterior to the celiac artery prior to injection.
It is beyond the scope of this chapter to describe every celiac plexus injection technique. The unilateral posterior paravertebral retrocrural technique (splanchnic sympathetic block)is presented as it is arguably the safest approach.
The patient is positioned prone and prepared and draped in a sterile manner. The C-arm is rotated obliquely to a position 15 degrees ipsilateral to the side of needle entrance at the L1 vertebral level. The skin entry site is anesthetized with a local anesthetic, and a 22-gauge, 6- or 8-inch needle (depending on the size of the patient) is passed through the skin and advanced until contact is made with the L1 vertebral body. The needle is then advanced under lateral visualization until the needle tip is positioned just anterior to the ventral aspect of the vertebral body.
The needle tip position is confirmed using anteroposterior and lateral fluoroscopic imaging. The stylet is removed and tubing is attached to the needle hub as well as the syringe containing the radiopaque contrast medium. The contrast is then injected under continuous fluoroscopy to assess contrast spread consistent with positioning of the needle tip in the retrocrural space and to ensure no vascular uptake. After confirmation of appropriate needle tip position the contrast syringe is replaced by a syringe containing 10 mL of a long-acting anesthetic, such as bupivacaine. In total, approximately 20–30 mL of a long-acting anesthetic is injected to block the greater, lower, and least splanchnic nerves.
Myriad side effects and complications can occur when performing a celiac plexus or splanchnic sympathetic nerve block, depending on the technique used. Side effects resulting from the sympathetic blockade include hypotension and diarrhea, which lead to vascular dilation and increased bowel motility, respectively. Therefore, patients should be well hydrated with intravenous fluids prior to a celiac plexus block, and an existing bowel obstruction is a contraindication for this procedure. Another contraindication is anticoagulation therapy.
The complications associated with a celiac plexus block include pneumothorax, nerve injury, bleeding, bowel injury, and infection. Other complications from a celiac plexus block using the anterior and lateral techniques include injury to the liver, stomach, small and large bowels, and pancreas; hemorrhage; infection; and fistula formation.
The literature regarding celiac plexus blocks is substantial when compared with that for other sympathetic injections. The evidence is strong with moderate quality of evidence for the use of celiac plexus blocks and neurolysis in the treatment of abdominal pain related to cancer.
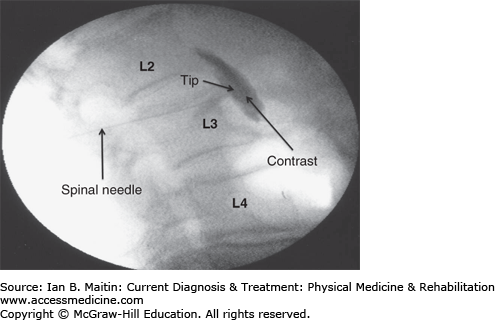
Lumbar sympathetic injections are typically performed in the treatment of acute and chronic neuropathic pain of the lower extremities resulting from disorders such as sympathetically maintained pain, complex regional pain syndrome, phantom limb pain, diabetic peripheral neuropathy, and herpes zoster, as well as for vascular insufficiency disorders. The lumbar sympathetic ganglia are located in front of the psoas muscle at the anterolateral inferior aspect of the L2 vertebra and at the anterolateral superior aspect of the L3 and L4 vertebrae.
Before a lumbar sympathetic injection is performed the patient should be hydrated with intravenous fluids to prevent hypotension as a result of vascular dilation. If there is bilateral involvement, the procedure is typically performed on one side at a time to prevent hypotension by limiting the injection to one lower extremity.
The patient is positioned prone on the procedure table and the area of the lumbar spine on the affected side is prepared and draped in sterile manner. The C-arm is rotated obliquely toward the affected side until the L3 transverse process is superimposed with the anterolateral aspect of the L3 vertebral body. The C-arm is then tilted until the superior endplate of L3 is squared off. Additionally, the C-arm may be tilted caudally or cranially if the transverse process is overlying the superior endplate of the L3 vertebral body to provide access to the superior endplate.
The skin overlying the needle insertion site is anesthetized with a local anesthetic. A 22-gauge, 6- or 8-inch spinal needle (depending on the patient’s body habitus) is inserted and advanced until contact is made with the upper outer corner of the anterolateral aspect of the L3 vertebral body. Once the spinal needle makes contact at the L3 vertebral body, the C-arm is positioned in a lateral view in order to advance the spinal needle under direct fluoroscopic visualization until the needle tip reaches the anterior portion of the L3 vertebral body. The use of a slight bend of the spinal needle tip helps the interventionist to more easily pass the spinal needle along the L3 vertebral body to reach the anterior portion of the L3 vertebral body in a lateral view.
The C-arm is repositioned in the anteroposterior position to confirm that the needle tip is located in the anterolateral position of the L3 vertebral body. Again, the C-arm is repositioned laterally and contrast is injected under continuous fluoroscopy to confirm contrast flow along the location of the L2, L3, and L4 sympathetic ganglia, as well as to ensure that there is no vascular uptake. The C-arm is then returned to the anteroposterior position to confirm contrast flow to the L2, L3, and L4 sympathetic ganglia (Figure 21–5). Finally, 20-mL of a long-acting anesthetic, such as bupivacaine, is injected to complete the lumbar sympathetic block.
Side effects and complications of lumbar sympathetic blockade include bleeding, infection, nerve injury, neuritis, visceral injury, hypertension, epidural or spinal block, paralysis, and anesthetic toxicity from intravascular injection.
The literature regarding lumbar sympathetic injections in the treatment of neuropathic pain is quite limited. Although there are many case reports, case studies, and technique papers in the literature, only two randomized studies have been published. One of these compared lumbar sympathectomy radiofrequency denervation to phenol neurolysis; the other was a randomized, controlled clinical trial that assessed chemical lumbar sympathectomy in the treatment of ischemic rest pain. Despite the low number of clinical studies, the results are strong with moderate quality for percutaneous lumbar sympathectomy in the treatment of neuropathic pain and ischemic pain using radiofrequency denervation or chemical neurolysis.
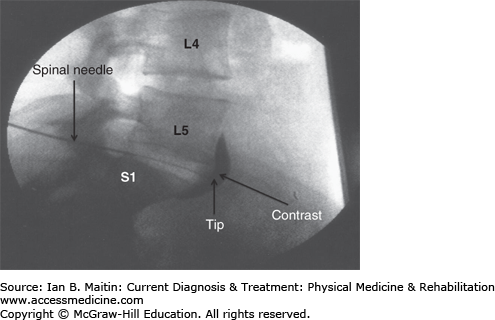
Sympathetic blockade of the superior hypogastric plexus is indicated in the treatment of lower abdominal and pelvic pain caused by a number of disorders, including pain of the genitalia, rectum, bladder, uterus, vagina, or prostate; endometriosis; and cancer or other chronic pain disorders involving the descending colon, sigmoid colon, and rectum. The superior hypogastric plexus is located anteriorly from the lower L5 to upper S1 vertebral bodies and below the bifurcation of the iliac vessels. Approaches for performing this block using a posterior single needle, posterior bilateral needle, anterior needle, and transintervertebral disc technique have been described (Figure 21–6). All techniques can be performed using fluoroscopic or CT guidance. The anterior approach has also been described using ultrasound guidance. The bilateral needle technique, also known as the classic technique, is described in this section.
The posterior bilateral needle approach is performed with the patient prone. The lumbosacral area is prepared and draped in a sterile manner. The C-arm is tilted caudally until there is a sharp anteroposterior view of the L5–S1 intervertebral disc. The C-arm is then rotated obliquely 45 degrees to one side. The skin overlying the insertion site is draped in a sterile manner. A 22-gauge, 6- or 8-inch spinal needle (depending on the patient’s body habitus) is inserted and advanced under fluoroscopic or CT guidance to the anterolateral aspect of the inferior L5 vertebral body. Next, the same steps are repeated on the opposite side to place the second needle tip at the anterolateral aspect of the contralateral inferior L5 vertebral body. Contrast is then injected under fluoroscopic guidance through both needles to confirm proper needle placement based on contrast flow, and to verify the absence of vascular uptake. Finally, 10 mL of a long-acting anesthetic, such as bupivacaine, is injected through each of the needles to block the superior hypogastric plexus.
The side effects and complications associated with sympathetic blockade of the superior hypogastric plexus depend on the technique employed to perform this procedure. With all techniques there is a risk of infection and bleeding, as well as bladder, rectal, and erectile dysfunction. In addition, the posterior single, posterior bilateral, and anterior needle techniques are associated with a risk of injury to vascular, nerve, and visceral structures. The transintervertebral disc approach appears to have little risk of the types of complications associated with these other techniques.
The literature contains few high-quality studies investigating superior hypogastric plexus sympathetic blocks and outcome. One prospective randomized trial, which compared the classic posterior approach with the transintravertebral disc technique, reported strong results and moderate-quality evidence. Both techniques produced the same degree of pain relief and morphine consumption trends throughout the followup period. However, there were some differences between the groups randomized to the classic posterior approach and the transverse vertebral disc technique. Two patients in the classic group did not receive any pain relief. Complications were reported in the classic group but not in the transdisc group. There was also a significant decrease in the procedure time for the transdisc group compared with the classic group.
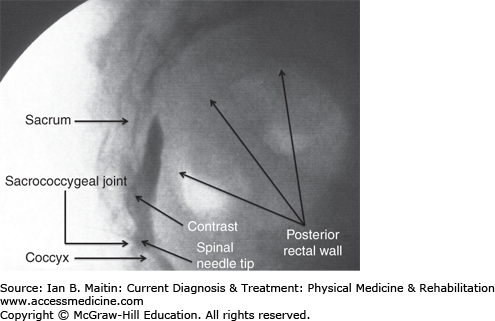
Impar ganglion sympathetic block is used in the treatment of perineal pain and coccydynia. The impar ganglion, also known as the ganglion of Walther, is located in the retroperitoneal space ventral to the sacrococcygeal joint. It is the last ganglion of the sympathetic chain found in the spine.
The original technique described for blocking the impar ganglion involved insertion of a spinal needle bent at an angle 5–7 cm proximal to the needle tip. The needle was first inserted inferior and anterior to the tip of the coccyx and then passed superiorly through the anococcygeal ligament to the level of the sacrococcygeal joint. This approach is technically demanding and has an inherent risk of injury to the rectum and surrounding vessels. The transsacrococcygeal technique presented below is easier to perform and safer than the original procedure, and provides equivalent blockade of the ganglion.
The patient is placed in the prone position and the sacrococcygeal area and buttocks are prepared and draped in a sterile manner. The C-arm is positioned with an anteroposterior view over the sacrococcygeal joint. The C-arm is then tilted in a caudal direction until the sacrococcygeal joint is squared off. The skin overlying the joint space is anesthetized with a local anesthetic, and a 22-gauge, 3½-inch spinal needle is passed through the skin until contact is made with the joint. The C-arm is then repositioned laterally after which the spinal needle is passed through the sacrococcygeal joint until the needle tip is anterior to the joint space. Contrast is injected outlining the retroperitoneal space between the ventral surface of the sacrum and coccyx posteriorly, and the dorsal aspect of the rectum anteriorly. Finally, 5 mL of a long-acting local anesthetic, such as bupivacaine, is injected to block the impar ganglion (Figure 21–7).
The impar ganglion block procedure described above is technically straightforward and rarely associated with any side effects or complications. As with all such procedures, bleeding and infection can occur, and puncture of the rectum is a rare occurrence.
Again, the literature contains few published reports of impar ganglion block procedures. Most of these consist of case reports. One prospective case series described 16 consecutive patients with chronic perineal pain who underwent impar ganglion blocks using the transsacrococcygeal method. The patients were followed over a period of 2 months, and there was a statistically significant reduction in Visual Analogue Scale (VAS) scores. The results of this study were considered strong based on low-quality evidence associated with its observational study design.
EPIDURAL LYSIS OF ADHESIONS
Epidural fibrosis with or without adhesive arachnoiditis is a possible complication of spinal surgery. The fibrosis can be caused by manipulation of the supporting structures of the spine, bleeding into the epidural space following surgery, or leakage of disc material. Epidural fibrosis is related to inflammatory reactions that result in the entrapment of nerves within dense scar tissue. Arachnoiditis is most frequently seen in patients who have undergone multiple surgical procedures of the spine. Presumably, inflammation and compression of nerve roots by epidural scar or fibrosis (adhesions) are the mechanisms underlying persistent pain following back surgery, a ruptured or herniated disc, or a vertebral body fracture.
Percutaneous epidural lysis of adhesions (also referred to as epidural neuroplasty or epidural adhesiolysis) has been developed as a conservative procedure to reduce or eliminate adhesions or fibrosis. A semirigid catheter with a flexible tip is placed into the epidural space to mechanically loosen or remove adhesions from the nerve roots. Hypertonic saline can be injected through the catheter at the area of fibrosis to mechanically disrupt adhesions and potentially reduce perineural edema. Hyaluronidase can also be injected to assist with breakdown of scar tissue and allow for better infiltration of a local anesthetic and corticosteroid mixture dispensed through the catheter at the site of nerve root involvement.
The indications for lysis of epidural adhesions include failed back surgery syndrome, chronic intractable back pain, and chronic radicular leg pain from a disc herniation. Local infection and sepsis are absolute contraindications to this procedure because of the potential for hematogenous spread via Batson’s plexus. Coagulopathy is another absolute contraindication because of potential compression of the spinal cord or thecal sac from a hematoma.
The patient is positioned prone and the sacral hiatus is identified by palpation and fluoroscopy. A 16-gauge, 3½-inch styletted needle suitable for catheter placement is inserted and advanced into the sacral hiatus. An anteroposterior fluoroscopic view is obtained to ensure that the needle tip is midline and positioned slightly toward the side of pain just below the S3 foramen. The location of the needle in the epidural space is verified by the injection of contrast under biplanar fluoroscopy, producing an epidurogram that also identifies the area(s) of adhesions.
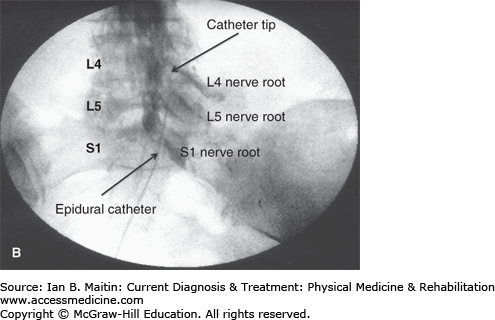
A styletted epidural catheter is used to perform the lysis of adhesions. To help steer the catheter in the epidural space, a small bend is made at the distal end of the stylet before it is reinserted into the catheter. The introducer needle stylet is withdrawn and the epidural catheter is carefully inserted through the needle to the level of the S3 sacral foramina. The catheter is steered gently by alternatively rotating the bent stylet from its proximal end and advancing or retracting the catheter to lyse the epidural adhesions under live fluoroscopy (Figure 21–8A). After mechanical lysis of the adhesions with the catheter, an additional 5–10 mL of contrast medium is slowly injected through the catheter to confirm the degree of adhesiolysis (Figure 21–8B). Hypertonic saline or hyaluronidase, or both, can be injected at this time through the epidural catheter to assist with the removal of scar tissue. A local anesthetic mixed with a corticosteroid is then injected after the lysis of adhesions through the catheter at the location of nerve root involvement to provide further therapeutic relief. The catheter is carefully removed after finishing the procedure so as not the shear any part of the catheter as it is withdrawn through the needle.
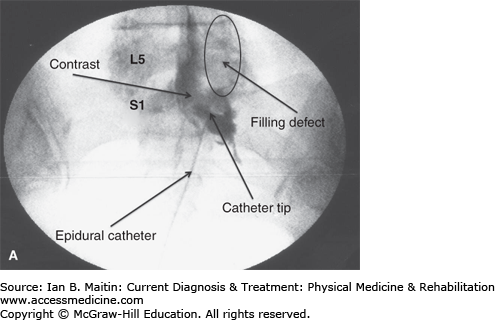
Figure 21–8
Epidural lysis of adhesions. A: Contrast injection revealing right-sided adhesions above the S1 nerve root represented by the filling defect. B: Contrast injection post–epidural lysis demonstrating elimination of adhesions with contrast flowing along the L4 and L5 nerve roots. L4, L4 vertebra; L5, L5 vertebra; S1, S1 vertebra.
Possible side effects and complications of this procedure include increasing pain in the injection site or worsening of symptoms, transient increase in back and leg pain, catheter fracture, and ecchymosis or hematoma formation over the sacral hiatus. More severe complications of epidural lysis of adhesions include local infections, sepsis, bleeding and hematoma formation causing compression of the spinal cord and paralysis, transient nerve compression with temporary paresis, unintended subdural or subarachnoid injection of hypertonic saline, persistent sensory deficit in the lumbar and sacral dermatomes, persistent bowel or bladder dysfunction (or both), and sexual dysfunction. Lysis of adhesions in the cervical or thoracic spine must be exercised with caution due to the significant risk for spinal cord trauma.
Epidural lysis of adhesions can reduce pain in 25% or more of patients who have lumbar radiculopathy plus low back pain refractory to conventional therapies for up to 1 year. Racz and colleagues reported that 65% of patients had therapeutic pain relief for 1–3 months but only 13% of patients had same pain relief for 3–6 months. Manchikanti and associates showed that there were no significant differences in pain relief among patients who underwent 1-day, 2-day, and 3-day procedures. In a prospective randomized, controlled study, Manchikanti and associates demonstrated long-term efficacy of pain relief from this procedure, with 97% of patients experiencing significant pain relief at 3 and 6 months, and 47% at 12 months. These patients also showed significant improvement in mental health and functional status, and a reduction in the use of narcotics. There appears to be no significant difference in treatment efficacy between normal saline and hypertonic saline or between hyaluronidase and hypertonic saline, although the use of hypertonic saline might reduce the number of patients who require additional treatments.
RADIOFREQUENCY NEUROLYSIS TECHNIQUES
The basic equipment needed to produce a radiofrequency (RF) tissue lesion from high-frequency waves includes a voltage generator, alternating current, and active and reference electrodes. The patient’s tissues serve as a resistor within the circuit and provide impedance. The active electrode is an insulated needle with an exposed tip, while the reference electrode is a large surface adhesive pad. This configuration leads to the greatest current concentration and heat being next to the tip, with diffusion of the current and heat at the large reference electrode. The current causes vibration of the electrons in the tissues near the RF probe, resulting in an increase in temperature. The greater the voltage and the tissue impedance, the higher the temperature that develops within the tissues.
The advantages of RF neurolysis include controlled lesion size, accurate temperature monitoring, limited need for anesthesia, precise probe placement, low incidence of morbidity or mortality, and rapid post-procedure recovery. The lesion size is dependent on the probe diameter, length of the uninsulated tip, temperature, time, and tissue vascularity. The lesion size is greater with a larger probe diameter, longer uninsulated tip, higher temperature, lower tissue vascularity, and longer lesioning time.
Pulsed RF neurolysis uses 10–30 ms bursts of high-frequency alternating current. Lesions created by this method are low temperature (cold RF) and nondestructive lesions. In producing an RF lesion the tissue that surrounds the tip of the electrode is exposed to an electromagnetic field.
Although the mechanism by which pulsed RF treatment works is not known, there are several theories. One theory is that the electromagnetic field may have a clinical neuromodulation effect rendering the nerve less likely to transmit painful impulses. Alternately, it may work in a manner similar to transcutaneous electrical nerve stimulation, activating both spinal and supraspinal mechanisms, which can reduce pain perception.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree