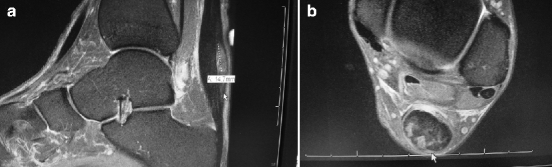
Fig. 14.1
(a) Retrocalcaneal exostosis lateral view. (b) “Zero degree” axial view showing lateral calcification. (c) Insertional tendocalcinosis
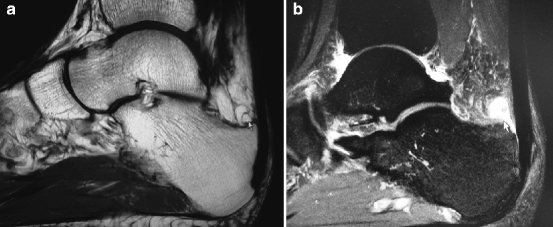
Fig. 14.2
(a) T1 MRI showing retrocalcaneal exostosis. (b) T2 MRI revealing bursitis
14.3.3 Surgical Technique of Insertional Repair/Retrocalcaneal Exostectomy
Surgical treatment involves removal of the exostoses, pathological bursae, remodeling of the posterior heel, excision of the insertional calcification (if present), and tenodesis of the AT with soft tissue anchors. The patient is placed in the prone position. Typically local anesthesia is used, often in conjunction with intra-venous sedation, but general and spinal anesthesia may be utilized. A tourniquet is typically not used but may be placed on the thigh or on the calf.
The incision is curvilinear from superomedial adjacent to the AT just above the superior calcaneus, inferiorly, across the posterior heel, staying within skin lines as much as possible, ending infero-lateral above the plantar skin lines (Fig. 14.3). The incision is deepened, and pathological bursae and the degenerated AT is excised. An inverted “T” approach to go “through” the AT insertion is used, exposing the superior calcaneus and any insertional calcification within the tendon insertion. The insertion calcification, if present, is excised (often with an osteotome), maintaining as much as the tendon expansion as possible. The superior calcaneus is further exposed after excising the retrocalcaneal bursa. This is resected with a curved osteotome from medial to lateral in both cases of retrocalcaneal exostoses (aka “pump bump”) and insertional calcification (aka “AITC”). A reciprocating rasp is helpful in smoothing off the rough edges and making a smooth rounded remodeled calcaneus (Fig. 14.4). After copious irrigation, bone wax can be placed on the superior surfaces from medial to lateral to help prevent ectopic bone formation (though this occurs in less than 5% of patients 4 or more years postoperatively).17
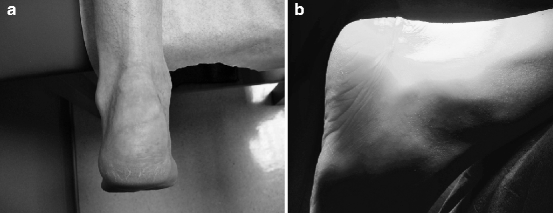
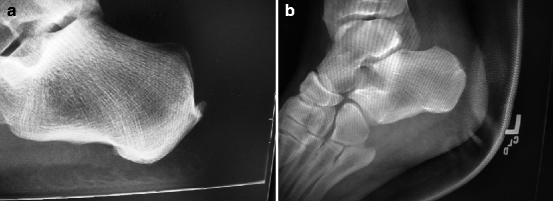

Fig. 14.3
Postoperative incisions showing (a) superomedial to infero-lateral approach and (b) transverse

Fig. 14.4
(a) Preoperative and (b) postoperative Achilles insertional repair with retrocalcaneal exostectomy/bursectomy
The AT is reattached with suture anchors. Generally the number of anchors used ranges from 1 to 43,6,8,9 (Fig. 14.5). More anchors are used when more of the insertion needs to be reattached. Absorbable anchors superiorly may be helpful, in case re-resection is needed.9,15 Care should be taken to place the suture knots in non-irritating regions. Irritation and granulomas from suture has recently been noted to occur in about 3% of AT surgeries in general.13 This can occur with both absorbable and nonabsorbable materials. The tendon proximally is repaired first, particularly in cases where tendon débridement is needed. After inferior reattachment with additional locking sutures, subcutaneous sutures with absorbable material are used. The skin is re-approximated with 3–0 nylon. A sterile compression dressing is applied, and the patient is placed in a splint or below-knee cast boot in slight equinus. Patients are seen within the first postoperatively week.
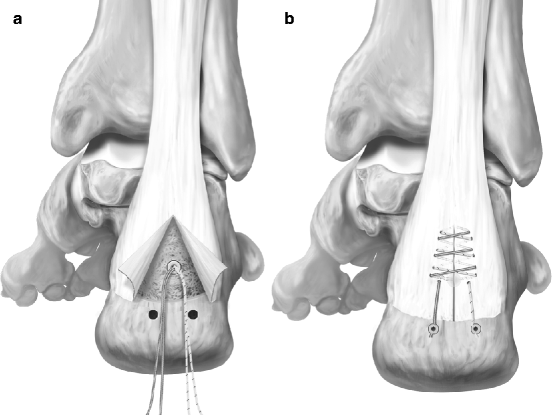

Fig. 14.5
(a, b) Suture anchors for Achilles tenodesis (Courtesy Arthrex, Inc., used with permission)
14.3.4 Postoperative Care
Patients are usually immobilized in a below-knee cast/boot non-weight bearing, for 4 weeks, followed by a weight-bearing period for an additional 6 weeks.3,9,15 Sutures are removed at 2 weeks. Patients are advised to take an oral anti-inflammatory (such as indomethocin 75 mg BID or naproxen sodium 500 mg BID) for 2 weeks post-surgery to prevent ectopic bone formation. Patients are advised to continue elevating and icing the limb for the entire postoperative recovery period. Active ROM is allowed at 3 weeks working on plantar flexion and inversion/eversion with a towel. Formal physical therapy is initiated around 8–10 weeks, though cross-training on a stationary bike is allowed with the boot/cast (again with the heel on the pedal) one week post-surgery while still in a cast/boot. Swimming is allowed (without flip-turns) at 6 weeks. Physical therapy includes progressive strengthening (initially with surgical tubing and/or a towel at 3 weeks) including single-legged heel raises. Return to daily activities occurs around 12 weeks; weight-bearing sport activities can take 16 or more weeks.9,15,18 Prior to using these surgical techniques, soft tissue anchors, and recommended period of immobilization, the results of this type of surgery were not as good as currently reported.
14.3.5 Conclusions
Retrocalcaneal Achilles insertional pathology can be relieved by surgery. Repair of the tendon insertion after debridement and calcaneal resection with soft tissue anchors appears to improve results. Patients should be advised of variability in postoperative convalescence.
14.4 Midsubstance Achilles Tendinopathy
Although scientifically sound epidemiological data are lacking, tendinopathy of the main body of the AT is common in athletes, accounting for 6–17% of all running injuries.19,20 However, it does present in middle-aged overweight nonathletic patients without history of increased physical activity.21,22 To date, no data are available to establish the incidence and prevalence of Achilles tendinopathy in other populations, even though the conditions has been correlated with seronegative arthropathies (e.g., ankylosing spondylitis).23
The essence of tendinopathy is a failed healing response, with degeneration and haphazard proliferation of tenocytes, disruption of collagen fibers, and subsequent increase in non-collagenous matrix.24 Tendinopathic lesions affect both collagen matrix and tenocytes. The parallel orientation of collagen fibers is lost; collagen fiber diameter and overall collagen density are decreased.
14.4.1 Diagnosis
The diagnosis of Achilles tendinopathy is mainly based on history and clinical examination. Pain is the pivotal symptom. A common symptom is morning stiffness or stiffness after a period of inactivity, and a gradual onset of pain during activity. In athletes, it occurs at the beginning and end of a training session, with a period of diminished discomfort in between. As the condition progresses, pain may occur during exercise and it may interfere with activities of daily living. In severe cases, pain occurs at rest. In the acute phase, the tendon is diffusely swollen and edematous, and tenderness is usually greatest 2–6 cm proximal to the tendon insertion. A tender, nodular swelling is usually present in chronic cases.
Clinical examination is the best diagnostic tool. Both legs are exposed from above the knees, and the patient examined while standing and prone. The AT should be palpated for tenderness, heat, thickening, nodule, and crepitation.25 The “painful arc” sign helps to distinguish between tendon and paratenon lesions. In paratendinopathy, the area of maximum thickening and tenderness remains fixed in relation to the malleoli from full dorsiflexion to plantar flexion; lesions within the tendon move with ankle motion. There is often a discrete nodule, whose tenderness markedly decreases or disappears when the tendon is put under tension.26 In the Royal London Hospital test, the clinician elicits local tenderness by palpating the tendon with the ankle in neutral position or slightly plantar flexed. The tenderness significantly decreases or totally disappears when the ankle is dorsiflexed.26
The clinical diagnosis of Achilles tendinopathy, even in experienced hands, is not straightforward, and experienced examiners may have problems in reproducing the results of clinical examination based on simple tests. If a patient presents with tendinopathy of the AT with a tender area of intratendinous swelling that moves with the tendon and whose tenderness significantly decreases or disappears when the tendon is put under tension, a clinical diagnosis of tendinopathy can be formulated, with a high positive predictive chance that the tendon will show ultrasonographic and histologic features of tendinopathy.26 In this instance, further imaging is indicated only for confirmatory, not diagnostic, purposes, as it is unlikely to change the management of the patient.26
14.4.1.1 VISA-A
The Victorian Institute of Sports Assessment – Achilles (VISA-A) questionnaire specifically measures the severity of Achilles tendinopathy.27 It covers the domains of pain, function, and activity. Scores are summed to give a total out of 100. An asymptomatic person would score 100. In clinical care, the VISA-A questionnaire provides a valid, reliable, and user-friendly index of the severity of Achilles tendinopathy. The VISA-A-S questionnaire showed good responsiveness in a randomized controlled trial (it was sensitive for clinically important changes over time with treatment, easy for the patients to fill out, and the data were easily handled).28 It has been cross-culturally adapted to Swedish,29 Italian,30 and Turkish.31
14.4.1.2 Imaging
Radiographs may be useful in diagnosing associated or incidental bony abnormalities. Radiographs are routinely obtained on patients with symptoms lasting longer than six weeks to rule out bony abnormalities, and identify the possible presence of intratendinous calcific deposits and ossification.
Ultrasonography, though operator-dependent, correlates well with histopathologic finding,32 and, especially in Europe, it is regarded as the primary imaging method. Only if ultrasonography remains unclear, MR imaging should be performed.33 A major advantage of ultrasonography over other imaging modalities is its interactive capability.34–36 Gray scale ultrasonography is associated with color or power Doppler to detect neovascularity.36
Magnetic resonance imaging (MRI) provides extensive information about the internal morphology of tendon and surrounding bone as well as other soft tissues. It allows to differentiate between paratendinopathy and tendinopathy of the main body of the tendon (Fig. 14.6). MRI is superior to ultrasound (US) in detecting incomplete tendon ruptures. However, given the high sensitivity of MRI, the data should be interpreted with caution, and correlated to the patient symptoms before making any recommendations.37
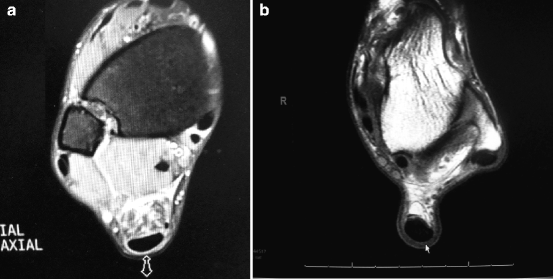

Fig. 14.6
(a) T2 and (b) T1 MRI showing paratendinosis with “halo-sign” (thickened paratenon)
14.4.2 Management
The management of Achilles tendinopathy lacks evidence-based support, and tendinopathy sufferers are at risk of long-term morbidity with unpredictable clinical outcome.38 The appropriate moment to switch from conservative to operative therapy remains unknown, as no solid data exist on the natural course of recovery. Nonoperative care should be in general a minimum of 3–6 months prior to considering surgery, since this condition has a good change of resolution. However, each patient should be evaluated independently.
14.4.2.1 Conservative Management
Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
Pharmacologic management strategies are essentially based on empirical evidence. Even though tendon biopsies show an absence of inflammatory cell infiltration, anti-inflammatory agents (nonsteroidal anti-inflammatory drugs and corticosteroids) are commonly used.41 What may appear clinically as an “acute tendinopathy” is actually a well-advanced failure of a chronic healing response in which there is neither histological nor biochemical evidence of inflammation.42 Ironically, the analgesic effect of NSAIDs43 allows patients to ignore early symptoms, possibly imposing further damage on the affected tendon and delaying definitive healing.44 NSAIDs appear to be effective, to some extent, for pain control. Early NSAIDs administration after an injury may have a deleterious effect on long-term tendon healing. Clearly there is a controversy on whether NSAIDs help or hinder the healing process.
Cryotherapy
Cryotherapy has been regarded as a useful intervention in the acute phase of Achilles tendinopathy, as it has an analgesic effect, reduces the metabolic rate of the tendon, and decreases the extravasation of blood and protein from new capillaries found in tendon injuries.45 However, recent evidence in upper limb tendinopathy indicates that the addition of ice did not offer any advantage over an exercise program consisting of eccentric and static stretching exercises.46
Eccentric Exercise
A program of eccentric exercise has been proposed to counteract the failed healing response which apparently underlies tendinopathy, by promoting collagen fiber cross-linkage formation within the tendon, thereby facilitating tendon remodeling.44 Although evidence of actual histological adaptations following a program of eccentric exercise is lacking, and the mechanisms by which a program of eccentric exercise may help to resolve the pain of tendinopathy remain unclear,47 clinical results following such exercise program appear promising.44,48 Though effective in Scandinavian population,48,49 the results of eccentric exercises observed from other study groups50,51 are less convincing than those reported from Scandinavia, with a 50–60% of good outcome after a regime of eccentric training both in athletic and sedentary patients. In general, the overall trend suggested a positive effect of an exercise program, with no study reporting adverse effects. Due to the lack of high-quality studies with clinically significant results, no strong conclusions can be made regarding the effectiveness of eccentric training (compared to control interventions) in relieving pain, improving function, or achieving patient satisfaction.39,47
In a randomized controlled trial51 the efficacy of three protocols – a “wait-and-see” approach, repetitive low-energy shock wave therapy, and eccentric calf strengthening – for the management of chronic tendinopathy of the main body of the tendo Achillis was compared. Spontaneous recovery after more than 6 months of symptoms of tendinopathy of the main body of the tendo Achillis was unlikely in the majority of patients. The likelihood of recovery after 4 months was comparable after both eccentric loading and shock wave therapy, as applied. Success rates were in the region of 60% with either of these management modalities.
Combined management strategies (eccentric training and shock wave therapy) resulted in higher success rates compared to eccentric loading alone or shock wave therapy alone in a recent randomized controlled trial.52 Eccentric training plus shock wave therapy should be offered to patients with chronic recalcitrant tendinopathy of the main body of the AT.52
Nitric Oxide
Nitric oxide is a small free radical generated by a family of enzymes, the nitric oxide synthases.53 Recently, a prospective, randomized, double-blinded, placebo-controlled clinical trial was performed in patients with tendinopathy of the main body of the Achilles to evaluate the efficacy of nitric oxide administration via an adhesive patch.54 Topical glyceryl trinitrate demonstrated efficacy in chronic noninsertional Achilles tendinopathy, and the treatment benefits continue at 3 years.55 However, a recent study from England56 failed to support the clinical benefit of topical glyceryl trinitrate patches.
14.4.2.2 Physical Modalities
The role of physical modalities in the management of tendinopathies remains unclear, and it is not possible to draw firm, evidence-based conclusions on their effectiveness.
The rationale for the clinical use of low-energy shock wave therapy to address the failed healing response of a tendon is the stimulation of soft tissue healing and the inhibition of pain receptors.51 Low-energy shock wave therapy and eccentric training produced comparable results in a randomized controlled trial,51 and both management modalities showed outcomes superior to the wait-and-see policy. The likelihood of recovery after 4 months was comparable after both eccentric loading and shock wave therapy, but success rates were 50–60%.
Hyperthermia induced by microwave diathermy raises the temperature of deep tissues to 41–45°C using electromagnetic power.57 Hyperthermia induced into tissue by microwave diathermy can stimulate repair processes, increase drug activity, allow more efficient relief from pain, help removal toxic wastes, increase tendon extensibility and reduce muscle and joint stiffness.57
Ultrasound therapy is a widely available and frequently used electrophysical agent in sports medicine. However, systematic reviews and meta-analyses have repeatedly concluded that there is insufficient evidence to support a beneficial effect of ultrasound at dosages currently being introduced clinically. A new direction for ultrasound therapy in sports medicine has been proposed by research demonstrating that ultrasound can have clinically significant beneficial effects on injured tissue when low-intensity pulsed ultrasound is used.58
14.4.2.3 Intratendinous Injection
14.4.2.4 Sclerosing Injections and Neovascularization
In patients with chronic painful tendinopathy of tendo Achillis, but not in normal pain-free tendons, there is neovascularization outside and inside the ventral part of the tendinopathic area.61,62 The good clinical effects with eccentric training may be due to the action on the neovessels and accompanying nerves. Also, local anesthetic injected in the area of neovascularization outside the tendon resulted in a pain-free tendon, indicating that this area is involved in pain generation. These are the bases for the injection of sclerosing substance polidocanol under ultrasound and color Doppler-guidance in the area with neovessels and nerves outside the tendon.
14.4.2.5 High Volume Ultrasound Guided Injections
High volume ultrasound guided injections aim to produce local mechanical effects causing neovessels to stretch, break, or occlude.63 By occluding and possibly breaking these neovessels, the accompanying nerve supply would also be damaged either by trauma or ischemia, therefore decreasing the pain in patients with resistant Achilles tendinopathy. In a pilot study,63 high volume image guided tendo Achillis injection of normal saline in patients with resistant Achilles tendinopathy decreased the amount of pain perceived by patients, while at the same time improving daily functional ankle and Achilles movements in the short- and long-term.
14.4.3 Surgery
14.4.3.1 Surgical Management of Tendinopathy of the Main Body of the AT
In 24–45.5% of patients with Achilles tendinopathy, conservative management is unsuccessful, and surgery is recommended after exhausting conservative methods of management, often tried for at least 6 months.64,65 There is a lack of trials on surgical management of Achilles tendinopathy, and therefore the high success rate needs to be interpreted with caution. Surgical options range from simple percutaneous tenotomy66,67 (possibly ultrasound-guided68), to minimally invasive stripping of the tendon,69 to open procedures (Fig. 14.7).
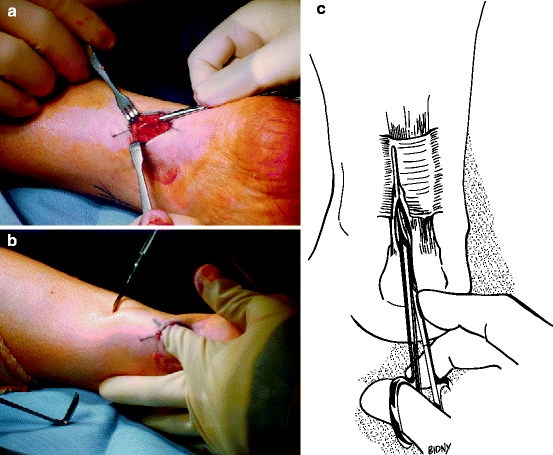

Fig. 14.7
(a–c) Intraoperative views and diagram of Achilles peritenolysis/decompression. Note thickened “watershed band.” Key to success of the procedure is to make sure all the constricting paratenon is removed. When passively dorsiflexing the ankle, there should be no “tenting” over the Achilles tendon in the watershed region
The classical aim of open surgery is to excise fibrotic adhesions, remove areas of failed healing and make multiple longitudinal incisions in the tendon to detect intratendinous lesions and to restore vascularity and possibly stimulate the remaining viable cells to initiate cell matrix response and healing45 (Fig 14.8). However, there is no level I evidence that fibrotic adhesions should be removed, and the areas of failed healing should be excised,66–68 at least if the pathology does not involve the paratenon. Multiple longitudinal tenotomies trigger well-ordered neoangiogenesis of the AT70 (Fig. 14.9). This would result in improved nutrition and a more favorable environment for healing.