CHAPTER 3 Indications for Unicompartmental Knee Arthroplasty
 Principal indications for medial UKA are anteromedial osteoarthritis and avascular necrosis (also called spontaneous osteonecrosis of the knee).
Principal indications for medial UKA are anteromedial osteoarthritis and avascular necrosis (also called spontaneous osteonecrosis of the knee). There should be “bone-on-bone” contact in the affected medial compartment with a functionally intact ACL and varus correctible if present.
There should be “bone-on-bone” contact in the affected medial compartment with a functionally intact ACL and varus correctible if present.Introduction
This chapter provides an overview of the indications and contraindications for unicompartmental knee arthroplasty (UKA), with specific reference to the Oxford UKA. The Oxford UKA has a fully congruent, freely mobile meniscal bearing that is free to slide and rotate between the congruent surfaces of the spherical femur and flat tibia, and this congruency is maintained in all positions throughout the range of movement of the knee joint.1 These unique design features help in minimizing wear2 and also make the implant “patella friendly.” Therefore, the indications outlined in this chapter have a specific reference to (or evidence for) the Oxford UKA, and generalization of all these indications for any other design of UKA may not be possible.
Indications
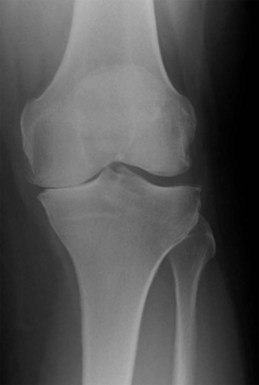
The principal indications for a medial Oxford UKA are anteromedial osteoarthritis (AMOA)3 (Fig. 3–1), and avascular necrosis (also known as spontaneous osteonecrosis of the knee, or SONK)1 (Fig. 3–2). AMOA, the most common indication for UKA, is a distinct entity, and it can be recognized by a consistent association between the clinicoradiologic signs and the pathologic lesions that cause them.1
Correlations
Intact cruciate ligaments and MCL can explain the symptoms and physical signs.1 Cruciate ligaments maintain the normal pattern of roll-back (“physiological roll-back”) of the femur on the tibia in the sagittal plane and thereby preserve the distinction between the damaged contact areas in extension (the anterior tibial plateau and the distal surface of the medial femoral condyle) and the intact contact areas in flexion (the posterior tibial plateau and the posterior surface of the femoral condyle). The shortened posterior capsule causes the flexion deformity. The varus deformity of the extended leg is caused by loss of cartilage and bone from the contact areas in extension. The angle of varus will depend on the amount of bone loss. To expose bone on both surfaces, the total thickness of cartilage lost is about 5 mm, causing about 5° of varus. At least this degree of deformity is usual on presentation because pain seldom becomes severe until there is bone-on-bone contact during weight bearing. Thereafter, each millimeter of bone eroded will increase the deformity by about 1°.
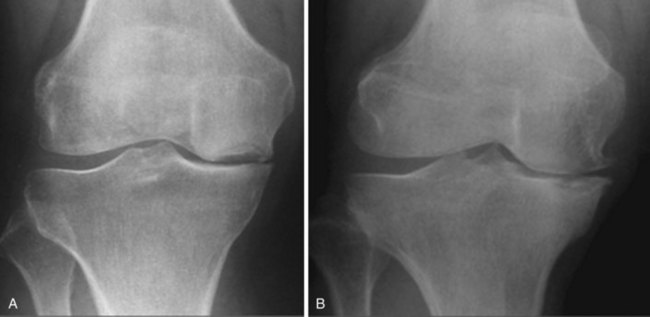
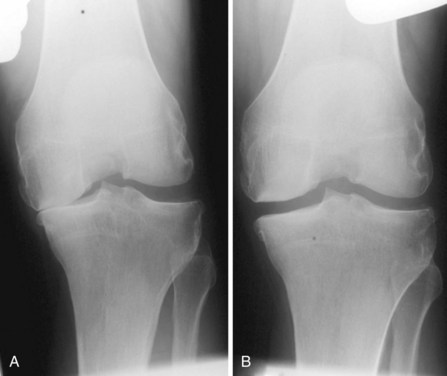
A diagnosis of AMOA is usually based on clinical findings as described above, although supportive evidence from radiographs is useful. Good-quality weight-bearing anteroposterior and lateral radiographs of the knee will help establish the presence of bone-on-bone appearance in the medial compartment and a varus deformity, which is usually present. If for some reason the radiograph does not confirm the presence of bone on bone in the affected medial compartment—that is, there is full-thickness cartilage loss (FTCL) over the femur as well as the tibia in the affected compartment—one can confirm the same by other investigations such as a varus stress view (Fig. 3–3A). In this view, the surgeon (or his or her assistant/radiographer) gives a varus stress to the knee under examination and takes an anteroposterior radiograph with the knee flexed to 20° to allow relaxation of the posterior capsule. After performing the varus stress radiograph, it is a good practice to obtain a valgus stress view (Fig. 3–3B). The valgus stress view allows confirmation of the presence of full-thickness cartilage in the lateral compartment, which is a prerequisite before proceeding to UKA. Some surgeons prefer to perform a Rosenberg view, which is equally useful in confirming the presence of FTCL in the medial compartment. If all these investigations fail to confirm the presence of FTCL in the affected medial compartment, the surgeon should perform an arthroscopy of the affected knee. If any of these investigations confirmed FTCL on both femur and tibia and the patient’s symptoms are bad enough to undergo knee replacement, then the surgeon can proceed to perform a UKA. If, indeed, this is not the case, then one should not perform UKA as the results are unreliable. We have not found other investigations (e.g., magnetic resonance imaging, computed tomography, or bone scan) to be of any specific value to confirm the presence of FTCL in the medial compartment; however, with improving imaging technology, this remains a possibility.
Anterior Cruciate Ligament
The anatomic state of the ACL at the time of surgery is an important determinant in the long-term outcome of UKA, as shown by Goodfellow et al. in 1992.1 They reported a sixfold difference in the 7-year cumulative survival of the Oxford UKA between knees with or without a functioning ACL at the time of surgery, irrespective of the primary disease and of all the other variables measured. In patients with AMOA, the ACL is invariably intact. White et al. described 46 medial tibial plateaus excised sequentially from a series of osteoarthritic knees treated by Oxford UKA, all of them with an intact ACL and with cartilage erosion exposing bone (Ahlbäck stages 2, 3, and 4).3 The erosions were all anterior and central. These rarely extended to the posterior quarter of the plateau and never reached the posterior joint margin. Similar findings have been confirmed by other investigators. Harman et al.4 examined the tibial plateau excised from 143 osteoarthritic knees during operations for total knee arthroplasty (TKA). They found that wear in ACL-deficient knees was located a mean 4 mm more posterior on the medial plateau than wear in ACL-intact knees. The ACL-deficient knees also exhibited more severe varus deformity. The site and extent of the tibial erosions can be reliably determined from lateral radiographs. Based on this, Keyes et al.5 studied the preoperative lateral radiographs of 50 osteoarthritic knees in which the state of the ACL had been recorded at surgery. Using four blind observers, they found a 95% correlation between preservation of the posterior part of the medial tibial plateau on radiograph and an intact ACL at surgery, and a 100% correlation of erosion of the posterior plateau on the radiograph with an absent or badly damaged ACL. These correlations show that, as long as the ACL remains intact, the tibiofemoral contact areas in flexion remain distinct from the areas of contact in extension. Progressive loss of bone causes the varus deformity in extension to increase but, while the ACL continues to function, this deformity corrects spontaneously in flexion and structural shortening of the MCL does not occur. If not treated in time, the deterioration observed in the ACL usually progresses via the following sequence1: normal → loss of synovial covering, → usually starting distally, → longitudinal splits in the substance of the exposed ligament, → stretching and loss of strength of the collagen bundles, which results in the ligament becoming “friable and fragmented.” The ACL will eventually rupture and disappear.
For the purpose of performing an Oxford UKA, we believe that, as long as the ACL is functionally intact (i.e., normal ACL or ACL with loss of synovial covering or longitudinal splits in the substance of the exposed ACL), an Oxford UKA may be safely performed. If the ACL is functionally impaired, this event will cause the transition from AMOA to the posteromedial form of the disease, with posterior subluxation of the femur and structural shortening of the MCL. Deschamps and Lapeyre6 observed that the absence of the ACL in an osteoarthritic knee was associated with the posterior subluxation of the femur on the tibia in extension. This subluxation results in the abrasion of the cartilage at the back of the tibial plateau by the exposed bone on the inferior surface of the femoral condyle. Thereafter, in flexion the cartilage on the posterior surface of the femoral condyle gets destroyed by abrasion on the tibial plateau, which is now devoid of any cartilage. The varus deformity is also therefore present in flexion as well as in extension and the MCL shortens structurally.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree