Fig. 1
Immunomagnetic selection of cells. (1) The biotinylated antibodies are bound to the positive cells. (2) The streptavidin-microbeads bind to the biotinylated antibodies. (3) The magnetic columns are placed in the magnet. The solution containing the antibody-bound single-cell suspension and the streptavidin-microbeads is passed through the column. (4) The complex formed by cells, biotinylated antibodies and streptavidin microbeads is retained on the columns, while the negative cell fraction is recovered. (5) Columns are separated from the magnet and the positive cell fraction can be eluted and recovered
1.
Calculate the amount of streptavidin-coated magnetic microbeads required, based on the number of cells and the capacity of the beads as indicated by the manufacturer.
2.
Transfer the desired volume of magnetic beads to a tube.
3.
Add the same volume of cold PBS + BSA + EDTA.
4.
Place the tube on a magnet for 1 min and carefully discard the supernatant by aspiration while the tube is still attached to the magnet.
5.
Remove the tube from the magnet and resuspend the washed beads in 1 ml of cold PBS + BSA + EDTA.
6.
Transfer the desired number of cells to a 15-ml conical tube.
7.
Centrifuge the sample for 10 min at 200 × g and 4 °C.
8.
Discard the supernatant.
9.
10.
Add 1–3 ml of cold PBS + BSA + EDTA.
11.
Centrifuge the sample for 10 min 200 × g and 4 °C.
12.
Discard the supernatant containing the unbound antibodies.
13.
Resuspend the cell pellet in the solution containing the optimal amount of washed streptavidin-coated magnetic beads (obtained in step 5 above) (see Note 21 ).
14.
Incubate for 15 min at 4 °C. Filter the dissociated tissue through a cell strainer with 30-μm pore size (see Notes 22 ).
16.
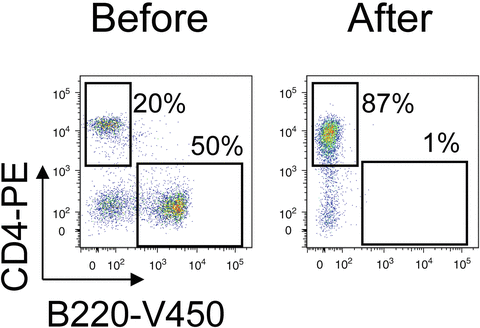
Recover the flow-through in a 15-ml conical tube. This fraction contains the cells that were not bound to the mixture of antibodies. In the case of negative selection, this fraction will contain the CD4+ T cells.
17.
18.
Add RPMI + FBS + Na-Pyr + 2-ME + Pen/Str to adjust cell concentration to 8 × 105 cells/ml.
3.6 Activation and Differentiation of Naïve CD4+ T Cells to Different Th Subsets
1.
Add PBS to the anti-CD3 antibody to reach a final concentration of 10 μg/ml.
2.
4.
Fill the wells with 200 μl of cold PBS to wash out the excess antibody. Invert the plates quickly to empty the wells and refill them with PBS. Repeat this step two or three times.
5.
Block the wells by adding 100 μl of 3 % FBS in PBS for 15 min at room temperature.
6.
To complete medium containing 1 μg/ml anti-CD28, add the appropriate mix of cytokines and antibodies for the differentiation to each Th subset, as follows.
(a)
For Th0 differentiation: anti-IFNγ (4 μg/ml) and anti-IL-4 (4 μg/ml) antibodies and cytokine IL-2 (10 ng/ml).
(b)
For Th1 differentiation: anti-IL-4 antibody (4 μg/ml) and cytokines IL-2 (10 ng/ml) and IL-12 (10 ng/ml).
(c)
For Th2 differentiation: anti-IFNγ antibody (4 μg/ml) and cytokines IL-4 (10 ng/ml) and IL-2 (10 ng/ml).
(d)
For Th17 differentiation: anti-IFNγ (4 μg/ml) and anti-IL-4 antibodies (4 μg/ml), and cytokines IL-6 (20 ng/ml), TGF-β (10 ng/ml), and IL-23 (10 ng/ml).
7.
Remove the blocking solution from the CD3-coated wells (step 5 above) and to each well add 2 × 105 cells and 200 μl of medium containing the corresponding mix of cytokines and antibodies.
8.
Incubate the cells at 37 °C, 5 % CO2 for 4–5 days.
9.
Check the cultures every 1 or 2 days. If the medium starts to turn yellow, replace it with fresh medium containing the corresponding antibody and cytokine mix but excluding the anti-CD3 and anti-CD28 antibodies.
10.
On day 5, centrifuge the plates for 10 min at 200 × g.
11.
Discard the supernatant by aspiration and resuspend the cells at 2 × 105 cells/ml in complete medium supplemented with IL-2 (5 ng/ml). Expand the culture for a further 2–3 days.
3.7 Quantification of Th Populations by Intracellular Staining
The percentage of each Th cell subset can be determined from the profile of cytokines they produce.
1.
Plate 200 μl of cell suspension (2 × 106 cells/ml) in each well of a 96 well plate and stimulate them with ionomycin (1 μM) and PMA (10 ng/ml) for 4–6 h at 37 °C in the incubator.
2.




Add Brefeldin A (3–10 μg/ml) for the last 4 h to block cytokine secretion to the medium. Incubate the cells with fluorophore-conjugated anti-CD4 antibody for 15 min and 4 °C.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree