Fig. 14.1
Regions and quadrants of the human abdomen
The upper abdomen is afforded protection from blunt trauma by the lower rib cage. An additional level of protection is provided to the abdominal viscera by the anterolateral abdominal wall consisting of skin, subcutaneous tissue, musculature, fascia, and parietal peritoneum [1]. The parietal peritoneum is a serous membrane lining the interior abdominal wall, and the visceral peritoneum surrounds the abdominal viscera. The thin space between the parietal and visceral peritoneum forms the peritoneal cavity. The kidneys, located posterior to the parietal peritoneum and bordering the posterior abdominal wall, are therefore classified as retroperitoneal.
Mesentery consists of two layers of peritoneum either extending between organs or connecting underlying viscera to the abdominal wall [1]. Mesentery provides vascular and neural connections to the abdominal organs and is essential to the mobility of the viscera within the abdominal cavity. The length of the tethering mesentery permits or restricts the relative motion of organs. Subdivisions of mesentery include the peritoneal ligaments extending between organs and omentum that connects the stomach to the surrounding viscera. The lesser omentum consists of the gastrohepatic and gastroduodenal ligaments and extends between the lesser curvature of the stomach and the liver. The greater omentum spans from the greater curvature of the stomach to cover the majority of the abdominal viscera and folds over to connect to the transverse colon. The sheet-like, fatty omentum separates the viscera from the parietal peritoneum and the abdominal wall, adding to the protection of the abdominal organs during blunt impact. The anatomical features of the abdomen are depicted in Fig. 14.2.
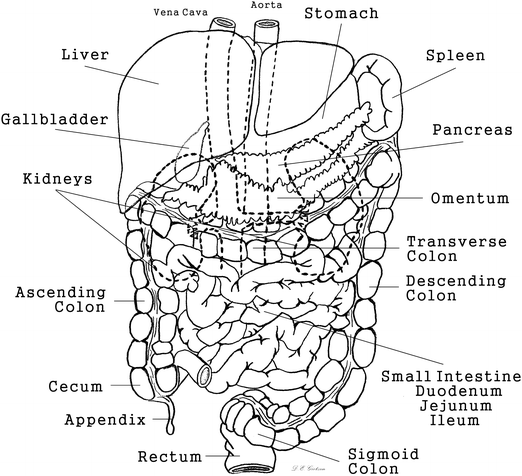

Fig. 14.2
Gross anatomy of the abdominal cavity
14.1.1 Hollow or Membranous Abdominal Organs
The hollow abdominal organs include the stomach, small intestine, large intestine, and gallbladder. The urinary bladder, a hollow, muscular organ, is commonly included in this classification. For the purposes of material testing, the diaphragm, mesentery, and vasculature are also categorized with the hollow abdominal organs as membranous soft tissues with similar biomechanical test protocols. The most common crash-induced injuries of the hollow abdominal organs are serosal tears, or lacerations, and perforations, or rupture-type injuries [2–5]. Therefore, the wall structure and the biomechanical response of the intact wall are essential to understanding the injury mechanisms and failure tolerances for accurately predicting hollow abdominal organ injury in blunt trauma.
Together the esophagus, stomach, and the small and large intestines make up the alimentary canal, or gastrointestinal tract, and perform the various aspects of digestion [6]. In general, the wall structure of the alimentary canal has a consistent layered architecture throughout its length. The four wall layers, described from the lumen to the outer layer, consist of the mucosa, submucosa, muscularis externa, and serosa or adventitia. The mucosa is organized in three concentric layers: an epithelial lining, a layer of connective tissue known as the lamina propria, and a smooth muscle layer, the muscularis mucosae. Nervous, vascular, and lymphatic connections reach the mucosa through the connective tissue of the submucosal layer. The muscularis externa is comprised of an inner circular smooth muscle layer bound in tight helices and a loosely bound outer longitudinal smooth muscle layer. The outer layer of the wall structure varies throughout the gastrointestinal tract. Structures suspended by peritoneum have an outer layer of serosa consisting of connective tissue and mesothelium, and structures attached to the abdominal or pelvic wall have an outer layer of connective tissue known as adventitia. Variations in the wall structure of specific organs are described in the respective sections.
14.1.1.1 The Stomach
The stomach is continuous superiorly with the esophagus and inferiorly with the proximal duodenum of the small intestine [1]. The organ itself is a hollow reservoir with four regions: the cardia, adjacent to the esophageal opening, the fundus, the corpus or body of the stomach, and the pyloric antrum. The lesser curvature extends from the cardia to the pyloric region on the medial side, and the greater curvature forms the lateral and inferior borders of the stomach. The inner mucosal layer of the stomach forms longitudinal folds, or rugae, that permit extension of the stomach during filling [7]. Rugae are less prominent in the fundus and upper region of the corpus, where the stomach wall generally appears more thin and smooth.
The stomach can accommodate a volume of up to 1.0 or 1.5 l of food or fluid [8]. Although the position and shape of the stomach change with the stages of digestion, the stomach generally extends from the level of the 5th left rib to the level of the 9th costal cartilage or the first lumbar vertebra [1]. The pyloric region crosses the midline of the body, where it is continuous with the duodenum to the right of the midline. The stomach is situated beneath the diaphragm, adjacent to the spleen, left kidney and adrenal gland, and pancreas, and lies posterior to the transverse colon.
14.1.1.2 The Small Intestine
The small intestine is divided into three regions: the duodenum, the jejunum, and the ileum [1]. The duodenum is approximately 23–28 cm in length, extending from the pylorus of the stomach to the duodenojejunal flexure, and wrapping around the head of the pancreas. The remainder of the cadaveric intestine is 6–7 m in length, extending from the duodenojejunal junction to the ileocecal junction. The jejunum and ileum are continuous but can be distinguished visually by a decrease in diameter and a change in wall thickness at the ileum. With the exception of the duodenum, the small intestine is extremely mobile within the abdominal cavity. Mesentery connects the jejunum and ileum to the posterior abdominal wall and provides the blood and lymphatic supply to the small intestine.
The wall structure of the small intestine is continuous throughout its length and consistent with the remainder of the gastrointestinal tract. As the three main functions of the intestine include mixing, propulsion, and absorption, mucosal folds appear as a result of contractions of the muscularis mucosae during mixing and propulsion, and the folds exist to increase absorption rates [8].
14.1.1.3 The Large Intestine
The cecum, colon, rectum, and anal canal make up the large intestine [1]. The colon is divided into four anatomic regions arcing around the border of the abdomen: the ascending, transverse, descending, and sigmoid colon. Both the ascending and descending colon are retroperitoneal, which provides some protection from blunt impact. The transverse colon is fairly mobile extending between the ascending and descending colon, with the curvatures between the regions known as the right and left colic flexure, respectively. The sigmoid colon curves from the descending colon, adjacent to the iliac fossa, to the rectum.
The wall structure of the colon matches the general structure of the alimentary canal and is consistent throughout the length [7]. From the lumen to the outer layer, the wall structure consists of the mucosa, submucosa, muscularis externa, and serosa. The distinguishing characteristics of the large intestine are teniae coli and haustra. Teniae coli are thin bands of muscle fibers extending axially throughout the large intestine to the rectum, and haustra coli are sections of tissue between the bands of teniae. During digestion, the proximal portion of the colon functions for absorption and the distal portion functions for storage of feces [8]. The contents of the colon, therefore, tend to be more fluid in the proximal portion and more solid in the distal half of the colon.
14.1.1.4 The Gallbladder
The gallbladder is a hollow reservoir that stores and concentrates bile produced in the liver and transported to the gallbladder through the common hepatic duct. In humans, the gallbladder can distend to accommodate approximately 50 ml [7]. The structure is surrounded by peritoneum, which fixes the body and neck of the gallbladder to the liver on the visceral surface. Concentrated bile is secreted from the gallbladder through the common bile duct to the duodenum of the small intestine. The wall structure of the gallbladder does not include a muscularis mucosae or submucosa layer, and it is the contractions of the smooth muscle cells in the muscularis externa of the lamina propria that empty the contents of the gallbladder into the cystic duct.
14.1.1.5 The Urinary Bladder
The urinary bladder is a round, hollow organ with thick, muscular walls. The bladder is located within the lesser pelvis when empty and extends toward the umbilicus during filling [1]. Two ureters open into the bladder, and the urethra extends to the external urethral orifice. The trigone is the triangular region formed by the three orifices of the bladder and has a consistent thickness [7]. The remainder of the bladder wall consists of thick, muscular folds arranged to direct urine toward the urethra during contraction.
14.1.1.6 The Uterus
The uterus is a thick-walled hollow organ positioned within the female lower pelvis between the bladder and the rectum. The uterus is divided into two regions: the body and the cervix [1]. The wall of the body consists of three layers: the outer perimetrium, a thick, muscular layer known as the myometrium, and the internal mucosal lining called the endometrium. The histology of the uterus varies throughout the menstrual cycle, and the position and structure alter considerably during pregnancy.
14.1.1.7 The Diaphragm
The diaphragm is a sheet of muscle and tendon that forms the superior border of the abdominal cavity [1]. The structure is mobile in the central region and fixed on the outer margins to the rib cage and lumbar spine with ligamentous attachments. The central tendon spans the central arc of the diaphragm and is surrounded by three regions of muscle fibers extending radially: the sternal and costal fibers, and the lumbar crura. The diaphragm arcs to form right and left domes. Three major diaphragmatic openings connect the thorax and the abdomen: the caval opening, the esophageal hiatus, and the aortic hiatus.
14.1.2 Solid Abdominal Organs
The solid abdominal organs include the liver, spleen, kidneys, and pancreas, and are frequent contributors to increased injury severity in automobile collisions. Injury distributions indicate that the liver and the spleen are the most frequently injured abdominal organs in motor vehicle collisions [9–11].
14.1.2.1 The Liver
The liver is the largest internal organ in the body, weighing up to 1,500 g and occupying the majority of the right upper quadrant of the abdomen [1]. The rib cage offers partial protection to the liver in blunt impact; however, blunt trauma can still occur and fractured ribs have the potential to result in penetrating trauma, or lacerations. Although damage to the liver is commonly described as “laceration”, in the absence of a true penetrating wound or broken rib and resulting cut or puncture, this damage is better described as “fracture”, which is a term frequently encountered in the biomechanics community in reference to solid organ disruption. Depending upon the extent of blunt trauma to the liver, the injury can be described as a “rupture” or “burst”, indicating extreme organ disruption, which is indicative of the highly vascular/fluid-filled nature of the liver.
The anterior, superior surface of the liver is known as the diaphragmatic surface due to the location inferior to the diaphragm [1]. The posterior, inferior surface of the liver is known as the visceral surface and is adjacent to the stomach, duodenum, colon, and right kidney. The ligaments of the liver are reflections of the peritoneum. The diaphragmatic surface is separated into right and left lobes by the falciform ligament connecting to the anterior abdominal wall. The coronary ligament suspends the liver by tethering it to the diaphragm. The layers of the coronary ligament give rise to the right and left triangular ligaments. The posterior surface of the liver is divided into four lobes: the left, right, caudate, and quadrate lobes.
14.1.2.2 The Spleen
The spleen is a lymphatic organ responsible for the breakdown of red blood cells [1]. The spleen is positioned in the left upper quadrant of the abdomen, adjacent to the left colic flexure. The diaphragmatic surface of the spleen is anterior and inferior to the diaphragm, and the medial hilum, the location of the splenic artery and vein, borders the tail of the pancreas. The gastrosplenic ligament and the splenorenal ligament connect the spleen to the greater curvature of the stomach and to the left kidney, respectively. The spleen is generally up to 12 cm in length and 7 cm wide and does not extend below the rib cage, which offers some protection from blunt impact. The parenchyma of the spleen is comprised of white and red pulp and is protected by a thin, but tough, capsule. The mode of splenic injury familiar to most is rupture.
14.1.2.3 The Kidneys
The kidneys , positioned in the retroperitoneal space, are encased in fat and therefore remain well protected from blunt impact injury. The kidneys generally extend from T12 to L3, with the right kidney positioned lower than the left due to the expanse of the liver [1]. The suprarenal glands are adjacent to the kidneys superiorly. The kidneys are responsible for urine production. The renal artery, renal vein, and ureter enter and exit the kidney at the renal hilum. The kidney is comprised of renal lobes, which consist of renal pyramids (part of the renal medulla) and their associated cortices. Other major constituents of the kidneys include the cortex, renal pelvis, and the calyces.
14.1.2.4 The Pancreas
The pancreas is a digestive gland located in the retroperitoneal space and situated between the spleen and duodenum on the left and right sides and posterior to the transverse colon [1]. From the medial to lateral edge, the pancreas is classified into four regions: the head, neck, body, and tail. The length of the pancreas extends across the vertebral column, potentially facilitating compression injuries. Although relatively rare in occurrence, the pancreas can rupture and result in the release of pancreatic enzymes into the abdominal cavity. Pancreatic injury usually occurs in conjunction with other injuries, and can be associated with bleeding.
14.1.3 Vasculature
The major vessels within the abdominal region are positioned adjacent to the vertebral column and are retroperitoneal in location. The arteries are efferent vessels, resistant to blood pressure with a muscular and elastic composition. The veins are afferent, thin-walled vessels with a high expansion capacity for containing blood volume.
14.1.3.1 The Arteries
The aorta enters into the abdominal cavity through the aortic hiatus, located at the posterior aspect of the diaphragm at the level of the T12 vertebra [1]. The abdominal aorta extends anterior to the vertebral column to L4, where the aortic bifurcation into the common iliac arteries occurs at the left of the midline. The left and right common iliac arteries branch into the internal and external iliac arteries before exiting the abdominal region. The branches of the abdominal aorta are paired, extending into visceral or parietal branches to the left and right of the midline, or unpaired, dividing into the celiac trunk, superior and inferior mesenteric arteries, and the median sacral artery.
The superior mesenteric artery branches from the anterior surface of the abdominal aorta and extends through layers of mesentery to supply the majority of the intestines [12]. Its divisions reach the length of the jejunum and ileum, cecum, ascending colon, and the proximal portion of the transverse colon. The inferior mesenteric artery branches from the inferior region of the abdominal aorta and supplies the remainder of the colon and the proximal length of the rectum.
14.1.3.2 The Veins
Blood from the abdominal viscera is transported back to the heart via the portal venous system entering into the inferior vena cava (IVC) through the hepatic veins [1]. The common iliac veins combine to form the IVC at the level of L5, posterior to the right common iliac artery. The IVC extends to the right of the vertebral column and aorta, entering into the thorax through the caval opening in the diaphragm. In addition to draining the hepatic portal system through the hepatic veins, the other tributaries to the IVC include the iliac, lumbar, renal, suprarenal, and phrenic veins [12].
14.2 Field Accident Data
Nahum et al. (1970) analyzed data from the accident files of the UCLA Trauma Research Group [13]. The files contained data on collisions of 604 vehicles produced in the 1960–1969 model years. There were 972 occupants involved in the collisions, of which 20 % were lap belted and 1 % were shoulder belted. The abdomen was injured in 3.5 % of all occupants. The authors calculated injury rates for the abdomen-pelvis area in association with specific contact points. In the 1960–1966 vehicles 19.8 % of the drivers received abdominal injuries which were associated with the steering system (55 %), side interior (22 %), “impact” (9 %), seatbelts (3.6 %), and other contacts. In the 1967–1969 vehicles, after the introduction of energy-absorbing steering systems, the abdominal injury rate decreased to 16.5 % of the drivers. The steering system association decreased dramatically to 21 %, side interior increased (33 %), “impact” (11 %), seatbelts (11 %), and the rest from other contacts. The severity of the abdominal injuries was moderate to fatal for 73 % of the 1960–1966 group, and 52 % of the 1967–1969 group.
Nearly one in ten (9.8 %) passengers had abdominal injuries in the 1960–1966 group, and 15.3 % in the 1967–1969 group. For the 1960–1966 group, these injuries had contact associated with the lower instrument panel (38 %), the side interior (23 %), “impact” (15 %), ejection (8 %), the A-pillar (8 %), the seatbelt (8 %), and other contacts. In the 1967–1969 group, the injuries were associated with contact to the lower instrument panel (30 %), the seatbelt (15 %), the upper instrument panel (10 %), side interior (10 %), and other contacts.
Rear seat occupants saw a reduction in abdominal injury rate from 12.5 % to 2.2 % when comparing the 1960–1966 and 1967–1969 groups, respectively. The associated contacts were: the seat back (57 %), side interior (29 %), and side glass (14 %) in the 1960–1966 group; and the seat belt (100 %) in the 1967–1969 group. Note, there was a change in abdominal injury severity from 71 % moderate to fatal injury in the 1960–1967 group, to 100 % minor injury in the 1967–1969 group.
In 1979, Danner and Langwieder [14] and Danner et al. [15] analyzed data from 2,545 pedestrian collisions with passenger cars investigated by German motor vehicle insurers. They found that 19.8 % of all pedestrian injuries, and 6.5 % of all serious pedestrian injuries (AIS 3+) were to the abdomen (only the head and tibia had greater frequency). Children and the elderly appeared to be overrepresented in terms of serious abdominal injury, with 22.7 % of all the serious injuries to children in the abdominal region (only head and femur had more), and 24.8 % of all serious injuries to the elderly in the abdominal region (only head and tibia had more).
Ricci (1980) analyzed data from the National Crash Severity Study (NCSS), which reported information for collisions in which the most severe injury occurred to an occupant of a towed passenger car [16]. The study dates were January 1977 through March 1979, and included a stratified sampling scheme in seven different areas of the US. The NCSS includes data on approximately 25,000 actual vehicle occupants, and over 106,000 occupants if weighted data is used. Ricci found that 3.8 % of all injuries in the NCSS data base were to the abdominal region. However, when severity was taken into account, injury to the abdomen was overrepresented, accounting for 8.3 % of all serious (AIS 3+), 29.9 % of all severe (AIS 4+), and 30.7 % of all critical (AIS 5+) injuries.
Hobbs (1980) reported on a 2-year in-depth investigation of collisions involving approximately 2,500 occupants in the United Kingdom [17]. He found that while less than 1 % of belted occupants were fatally injured, 8.4 % of all unbelted occupants were fatally injured. Half of 1 % (0.5 %) of the belted occupants, and 1.7 % of the unbelted occupants received serious (AIS 3+) abdominal injuries in pure frontal and oblique frontal impacts. For injury of all severities, contact was associated with the seatbelt, instrument panel, and side door for belted occupants, and the steering system, instrument panel, and side door for the unbelted occupants. For rollovers, none (0 %) of the belted occupants and 3 % of the unbelted occupants sustained serious abdominal injuries.
Galer et al. (1985) studied 261 collisions in the UK which involved 297 vehicles and 506 occupants [18]. They analyzed the data for belted occupants, and found that 14.6 % of all injuries to drivers and 17.8 % of all injuries to front seat passengers were to the abdomen/pelvis (they did not examine the abdomen alone). These represented 6.4 % of the nonminor (AIS 2–6) injuries to drivers and 18.1 % of those to front seat passengers. The main contacts associated with minor injury (AIS 1) to the abdomen/pelvis of vehicle drivers were seatbelt webbing (59 %) and the side door (12 %), and those associated with nonminor injury were the steering system (30 %) and side door (70 %). For front seat passengers seatbelt webbing was the principal contact for minor injury (91 %), while the side door (50 %) and other vehicle contacts (50 %) were associated with nonminor injury to the abdomen/pelvis.
Rouhana and Foster (1985) analyzed the NCSS data (the same data set as Ricci [16]) specifically for side impact collisions [19]. They found that 15.6 % of all serious (AIS 3+) injuries, and 24.2 % of all severe (AIS 4+) injuries were abdominal. The main contact points associated with serious abdominal injury in left side impacts were the side interior (39 %), the armrest (30 %), and the steering system (18 %). The main contacts associated with serious abdominal injury in right side impacts were the glove compartment (39 %), side interior (28 %), and the armrest (28 %). The curious appearance of the glove compartment as an injury factor in side impacts reflects the large percentage of struck cars with forward velocity at the time of impact. The occupants on the side opposite the impact continue moving forward in those cases as described by Newton’s First Law.
Using the concept of “severity weighted frequency” of injury, the abdominal organs seriously injured for drivers in left side impacts were ordered as kidneys (4.5 %), liver (4.0 %), spleen (3.7 %), digestive (2.0 %), and urogenital (0.8 %). For passengers in right side impacts, the injuries were ordered as liver (7.0 %), spleen (3.2 %), kidneys (2.5 %), urogenital (2.5 %), and digestive (1.2 %). The near doubling of the proportion of liver injuries in right side impacts when compared to left side impacts makes sense from anatomical considerations.
Bondy (1980) also analyzed the statistics in the NCSS database [9]. This analysis showed that in frontal impact the order of serious abdominal injury was liver (39 %), spleen (25 %), digestive (16 %), kidney (14 %), and urogenital (2.6 %). The contact points associated with serious abdominal injury were the steering system (51 %), side interior/armrest (26 %), instrument panel/glove compartment (17 %), front seat back (5 %), and belt webbing (1 %). It is interesting to note that the restraint system was not associated with injury to any of the solid abdominal organs.
Mackay et al. (1993) studied the 1983–1989 side impact crash experience in the Birmingham region of the United Kingdom [20]. This study provides an interesting contrast to that by Rouhana and Foster [19] because, while the database used by Rouhana and Foster contained mainly unbelted occupants, the study by Mackay et al. contained only belted occupants. They found that 41 of the 180 occupants (23 %) in the study received an injury to the abdomen and the vast majority (71 %) of those injuries were associated with seatbelt loading. However, they found only five cases of abdominal injury with AIS 3+. Thus, as belt use rates in the US increase, one would expect the injury pattern for farside occupants in lateral impacts in the US to change from more serious injuries associated with interior structures in front of the occupant to mainly less serious injuries associated with the seat belt. In fact, using the National Automative Sampling System – Crashworthiness Data System (NASS-CDS) database from 1993 to 2002, Gabler et al. (2005) found that in farside impacts with belted occupants, only 6.8 % sustained AIS 3+ abdominal injuries and 86 % of AIS 2+ abdominal injuries were attributed to contact with the seatbelt or belt buckle [21].
Klinich and Burton (1993) analyzed data from the National Automotive Sampling System (NASS, formerly National Accident Sampling System) for the years 1988–1990 [22]. They studied injury to older children, defined as ages 6–12 years old. These data indicate that the frequency of serious injury (AIS 3+) to the pelvis and abdomen ranked fourth after head, lower extremities and thorax. They showed that while 62.4 % of restrained older children and only 36.4 % of unrestrained older children remain uninjured, the percentage of injuries to the abdomen and pelvis are 10.8 % and 2.6 %, respectively. They did not break this down further to examine injury severity which is typically lower when restrained.
Khaewpong et al. (1995) performed a study of 103 cases of restrained children who were admitted to the level I trauma center at the Children’s National Medical Center (CNMC) [23]. They studied the correctness and appropriateness of the restraint and the injuries sustained by the children. There were 12 cases of abdominal injury with maximum AIS 3+ to these restrained children. While the sample size was very small, several conclusions could be drawn relative to abdominal injury. Nearly 90 % of the abdominal injuries in this group were associated with contact with the restraint system. However, all of the children who sustained abdominal injury were using the restraint system either incorrectly, inappropriately, or both incorrectly and inappropriately.
Another very interesting observation was noted but not discussed in this paper. When the authors compared their results from the CNMC to results from a similar analysis of NASS data, they found that 52 % of the abdominal injuries to restrained children in the NASS data were to children in convertible child seats. They did not go into detail relative to injury or collision severity. Abdominal injury to children in seats with crotch straps seems unlikely to be more than AIS 1, so a more in-depth look at this data appears warranted.
Elhagediab and Rouhana (1998) reviewed abdominal injuries in non-rollover frontal impacts to non-ejected drivers and right front passengers [10]. They used the NASS database for the years 1988–1994. They found that abdominal injuries constituted 8 % of all injuries of AIS 3+, 16.5 % of all injuries of AIS 4+, and 20.5 % of all injuries of AIS 5+. The organs injured most frequently were the liver (38 %), spleen (23 %) and digestive system (17 %). The objects within the vehicle that were most often associated with the abdominal injuries were the steering wheel (68 %), belt (17 %) and airbag/other interior objects (14 %). Of note was the fact that only 10 % of the vehicles in the study contained airbags. In addition, right front passengers appeared to be more at risk of abdominal injury when restrained by lap-shoulder belts (12.5 %) than unrestrained (10.7 %). This statement, however, does not reflect the head, neck and thoracic injuries prevented by the lap-shoulder belt.
Yoganandan et al. (2000) examined abdominal injury frequency and severity in frontal, nearside, and farside impacts using the NASS-CDS database for the years 1993–1998 [24]. For a combined dataset of belted and unbelted drivers and right front passengers, the distribution of organ injury frequency for each crash mode varied when comparing AIS 2+ and AIS 3+ injuries. The organs injured most frequently in frontal crashes were the liver (39 %), spleen (29 %), and digestive organs (11 %) for AIS 2+ and the liver (35 %), spleen (28 %), and arteries (14 %) for AIS 3+. For nearside crashes, the spleen (49 %), kidneys (20 %), and liver (16 %) were injured most frequently for AIS 2+, and the spleen (47 %), diaphragm (17 %), and liver (13 %) were the most frequently injured organs for AIS 3+. The injury frequency for farside crashes was kidney (38 %), liver (32 %), and digestive (15 %) for AIS 2+ and liver (50 %), spleen (13 %), and kidney (12 %) for AIS 3+. The spleen was the most frequently injured organ in left side impacts, whereas the kidneys and liver were injured more frequently in right side impacts. The kidneys are protected by fat encasements and their retroperitoneal position; therefore, renal injuries tended to be less severe and comprised a greater percentage of AIS 2+ injuries in side impacts. Similarly, injury to the digestive tract is typically less severe on the threat-to-life scale, and therefore these injuries comprised a greater percentage when considering AIS 2+ abdominal injuries in frontal and farside impacts.
Lee and Yang (2002) characterized abdominal injury patterns in a similar dataset, using the NASS database from 1993 to 1997 [25]. The study focused on injury patterns for the solid abdominal organs, finding these injuries to account for 35 % of all abdominal injuries, with 32 % of these injuries AIS 3+. Lacerations were the most common injury type for the solid organs. Liver injuries were most frequently attributed to contact with components in the front of the occupant compartment, such as the steering assembly. Spleen injuries were most commonly attributed to contact with the left side of the occupant compartment.
Lamielle et al. (2006) analyzed abdominal injury patterns in frontal crashes occurring in France from 1970 to 2005 [26]. The likelihood of AIS 3+ abdominal organ injury increased with increasing crash severity. Injury risk was reduced with the use of seatbelts with retractors. Occupants with AIS 3+ abdominal injuries were significantly older than occupants without an AIS 3+ abdominal injury. This study identified belted rear seat occupants as more likely to sustain abdominal injury compared to belted front seat occupants. For belted occupants, hollow abdominal organ injury occurred more frequently (59 %) than solid organ injury, whereas solid abdominal organ injury occurred more frequently for unbelted occupants (77 %). The organs injured most frequently (AIS 3+) were the spleen (22 %), jejunum (16 %), and the liver (16 %).
Klinich et al. (2010) used the NASS-CDS (1998–2008) and Crash Injury Research and Engineering Network (CIREN) databases to assess abdominal injury in frontal, farside, and nearside crashes for a population of occupants in which 85 % were belted and 68 % were travelling in airbag-equipped vehicles [11]. The analysis emphasized characterizing injury for occupants utilizing current safety technologies. For all crash modes, belted occupants had a significant reduction in risk of AIS 2+ and AIS 3+ abdominal organ injuries compared to unbelted occupants. No difference in injury incidence was found in frontal crashes with and without airbag deployment. Right front passengers in nearside impacts had the highest risk of sustaining AIS 2+ abdominal organ injury. The percentage of occupants sustaining AIS 2+ abdominal organ injury, as well as the percentage of occupants with rib fracture, increased with increasing crash severity for all crash modes. Controlling for crash severity, the authors found that the likelihood of sustaining AIS 2+ and AIS 3+ injuries to the solid abdominal organs (liver, spleen, kidneys) increased considerably with the presence of AIS 2+ rib fractures. No association was found between abdominal organ injury incidence and occupant age.
Frampton et al. (2012) investigated abdominal injury risk factors for belted front and rear seat occupants in frontal crashes using crash data from the UK Co-operative Crash Injury Study (CCIS) from 1998 to 2010 [27]. The authors found an increased risk of AIS 2+ and AIS 3+ abdominal injuries for rear seat passengers compared to right front passengers and drivers. These results were consistent with the results of Lamielle et al. [26]. Abdominal injury risk increased with age for both severity levels; however, the injury incidence was also high for occupants less than 19 years of age. The most frequently injured organs for drivers were the liver and spleen, and for right front passengers, the most frequently injured organs were the liver, jejunum-ileum, and spleen. Rear seat passengers sustained a different injury pattern with the jejunum-ileum, mesentery, and the colon injured more frequently than the solid organs, though liver and spleen injury incidence remained high for rear seat occupants.
The association between abdominal organ injury and multiple rib fractures was also assessed. The majority of liver, spleen, and mesentery injuries were sustained in association with two or more rib fractures, whereas the majority of the colon, duodenum, and jejunum-ileum injuries were associated with one or no rib fractures. The incidence of rib fractures did not differ significantly between seating positions while the distributions of organ injury varied considerably for front and rear seat occupants.
To further the understanding of clinical data, Campbell et al. (1994) compared the Injury Impairment Scale (IIS) scores with physicians recorded estimates of impairment for 7,502 patients in the United Kingdom Major Trauma Outcome Study [28]. The IIS is an estimate of the impairment a patient will experience 1 year post-injury. The injuries had been sustained in motor vehicle crashes for 19.9 % (1,483) of the patients in the study. They found a strong, statistically significant correlation between IIS and physician’s estimates. They also observed that 99.5 % of all patients with abdominal injuries had an IIS score of 0 (no impairment) and only 1 patient was expected to have a permanent impairment. In contrast, about 8 % of all patients with head injury were expected to have permanent major impairment. Notably, these data reflected patients who had sustained a single injury to the head, abdomen or lower limbs. Information on impairment adds another dimension beyond the traditional and more immediate AIS threat-to-life scale.
These field accident studies have shown that blunt abdominal trauma is a common result of motor vehicle related collisions. As injury severity increases, abdominal organ injuries account for a greater percentage of all injuries. In general, there is a preponderance of abdominal injuries to the solid organs compared to hollow organs. The presence of AIS 2+ rib fractures is correlated with an increased risk of AIS 2+ abdominal organ injury. When age is accounted for, children and the elderly are overrepresented in terms of incidence of serious abdominal injuries. The order of organs injured is significantly different when comparing side and frontal impacts.
The contact points associated with injury to unbelted occupants include many of the structures within the vehicle (steering system, side door, instrument panel, etc.). While seatbelt use is associated with an increase in minor abdominal injury compared to the unbelted occupant, there is a clear decline in serious injury to the abdomen when seatbelts are used.
14.3 Clinical Data
Clinical studies of patients with blunt abdominal injury have identified Motor Vehicle Collision – Related incidents to account for 50–90 % of cases [29–32]. Abdominal injury distribution by organ varies in frequency and order depending on the inclusion criteria for the clinical studies. Historically, however, the solid abdominal organs (liver, spleen, kidneys) have been the most frequent contributors to blunt abdominal injury as well as injury severity. Although the hollow abdominal organs are injured less frequently in blunt trauma cases, high morbidity and mortality are associated with these injuries if not diagnosed [33].
Increases in the morbidity and mortality of abdominal injuries resulting from blunt trauma are often attributed to delayed diagnosis resulting from lacking or masked symptoms [33, 34]. However, improved evaluation techniques have considerably facilitated the diagnosis and treatment of abdominal injury, beginning with the introduction of diagnostic peritoneal lavage (DPL) as a simple and highly sensitive method compared to physical examination [35]. Further diagnostic advances including computed tomography (CT) and ultrasonography (US) have improved the ability to detect injuries with the advantage of being non-invasive [36–39]. While CT requires increased time for evaluation, for stable patients it can provide more specific information than DPL regarding which organs are injured, injury severity, and need for surgery. Traditional US and the focused abdominal sonography for trauma (FAST) screening exam have largely replaced DPL due to speed and simplicity [40]; however, US can be limited in the ability to detect free fluid and can fail to provide direct visualization of solid parenchymal or hollow visceral injuries [33, 39]. Despite limitations, these techniques have improved the evaluation of intra-abdominal injury.
Improved diagnostic methods, as well as multi-modality approaches, have helped physicians to develop nonoperative trauma management protocols, which appear to be quite effective, for stable patients with suspected abdominal trauma [41, 42]. Nonoperative management of liver injuries has shown increased effectiveness compared to splenic or kidney injuries [43]. Diaphragmatic injuries and hollow organ injuries continue to be a concern [44]. Due to the associated morbidity, reducing non-therapeutic laparotomy for these and other injuries remains as a goal for the management of abdominal trauma.
14.4 Material Properties Testing
Material properties testing can provide valuable insight in to the local response of the organ system, how a tissue fails, and at what level (stress or strain) it fails. Equibiaxial stretch testing of membranous tissues provides the response characteristics of components of the hollow organs using loading conditions and rates commensurate with those to be expected in a car crash. Dog bone, slug, or intact organ testing provides the response characteristics and material properties of solid organ structures. Common to all tissue testing are the issues of controlling and quantifying the initial and boundary conditions. Recreating the baseline (in-vivo or neutral) conditions of the tissue as a starting point is challenging. Depending upon the structure, this does not always correspond to a state of zero stress. For example, testing a tubular structure as a flat sample might result in the measurement of properties that are different than those that the tissue might exhibit in vivo.
Measurement of strain in a sample is certainly a challenge, but high-resolution, high-speed video facilitates the quantification of dot or speckle patterns. Measurement of stress is more difficult, as the continual change of tissue thickness must be quantified. Video and laser systems help in this regard, but often some assumptions must be made as to the distribution of load through a sample, and whether or not a tissue is incompressible.
14.4.1 Membranous Tissues
Limited material properties data are available in the literature for the complex structures of the digestive system. These data were obtained in uniaxial tension at quasi-static rates [45–48]. No historical data exist characterizing the dynamic biaxial material and failure properties of the human post-mortem hollow abdominal organs. The planar equibiaxial test configuration creates a two-dimensional nearly homogeneous strain field and incorporates the effects of tissue anisotropy in the response to multidirectional stretch. This experimental technique was applied to fresh human cadaver tissue including stomach, small intestine, and colon samples using a custom equibiaxial tissue-testing device. Mason et al. (2005) provided a detailed description of the components of the original device [49]. Therefore, the design and function of the device are explained only briefly.
The dynamic equibiaxial testing device was designed to apply simultaneous equal stretch to a cruciate-shaped tissue sample in four directions, as shown in Fig. 14.3. Sample arms were gripped in four low-mass tissue clamps designed to reduce inertial effects and limit slip between the sample and clamp. Each clamp was mounted to a lightweight carriage riding on linear rails with low-friction bearings. Carriages were individually coupled to a central drive disk by rigid links. Rotation of the disk has been generated by a pneumatic cylinder coupled to the test device [50], as well as by a hydraulic actuating mechanism rigidly linked to the device and loaded by a falling mass [51]. Rotation of the disk produced identical motion of the four carriages away from the disk center and orthogonal to the adjacent carriages. Miniature load cells and accelerometers were mounted to each clamp and carriage assembly. Laser displacement sensors were mounted above and below the sample to obtain changing sample thickness throughout the duration of the test. A high-speed video camera was mounted with the lens parallel to the tissue sample surface to obtain optical data without interfering with the test.
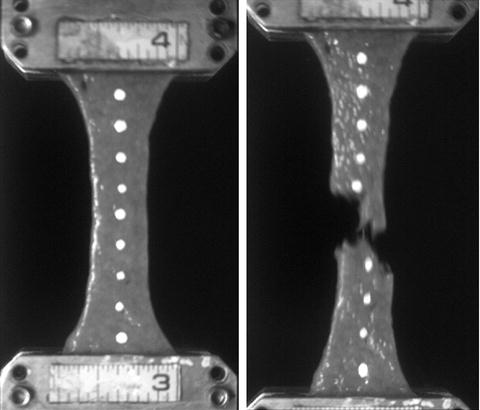
Howes and Hardy (2012) conducted tissue testing using samples harvested from the abdominal cavities of fresh, never-frozen post-mortem human surrogates [51]. A CNC-machined stamp was applied to tissue sections from each excised organ of the digestive tract, producing cruciate-shaped samples with four arm branches converging to form a 10-mm central square region of interest (ROI). The stamp was aligned such that two co-linear arms were oriented with the visible longitudinal fiber direction of the tissue, or offset from the fiber direction by 22° [52]. The offset prevented failure across the arm branches of the sample [50], resulting in failure through the central ROI such that local strain calculated in the ROI was a direct measure of the failure strain. Samples were stretched until complete transection occurred.
High-speed video data were analyzed using tracking software to quantify the displacement of ink markers applied to the ROI of the sample. Average directional Green-Lagrange strain, maximum principal strain and strain rate, and maximum shear strain were calculated. Applied load in the ROI was calculated by scaling each of the inertia compensated measured loads, assuming a proportional transmission of load through each arm to the central region. The two compensated scaled loads in each direction were averaged to provide the load time histories in the x- and y-directions. True stress was calculated using the laser thickness data to determine changing cross-sectional area throughout the duration of each test. The 2nd Piola Kirchhoff stress was calculated using the applied load, initial cross-sectional area, and the inverse of the stretch ratio. Stress data were filtered using SAE J211 CFC 600 Hz [53]. All data were transformed to align with the material axes of the tissue and truncated at the time of tear initiation.
Considering all of the hollow organ tests, which were conducted at a maximum principal strain rate of 72 s−1 on average, the average failure strain (including both directions) was approximately 15 %, and the average failure stresses were on the order of 2 to 4 MPa. Figure 14.4 shows exemplar stress vs. strain responses for three colon samples. Directional differences in failure properties were observed throughout the length of the digestive tract with an overall trend toward an increased stiffness in the longitudinal direction for the intestines. This analysis quantified the material properties and failure thresholds of the hollow abdominal organs loaded in equibiaxial tension at impact-level rates, with application to the optimization of material model parameters to represent abdominal tissue response and to the advancement of finite element models of the human abdomen.
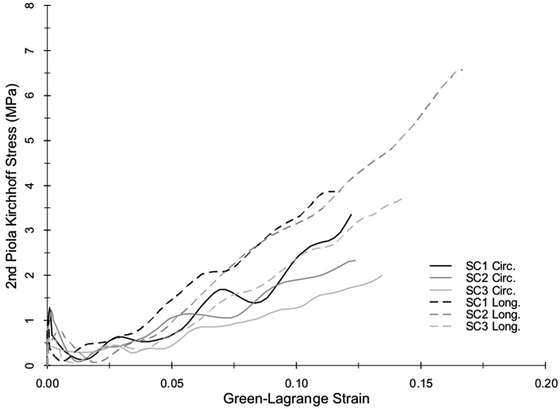

Fig. 14.4
Exemplar stress-strain responses for three cadaver colon samples undergoing high-rate equibiaxial stretch, from the research of Howes
14.4.2 The Liver in Tension
There have been a number of studies that have investigated the tensile material properties of liver by performing tension tests on isolated liver samples. In the interest of brevity, the summary of previous studies provided in this section is limited to studies that have reported tensile failure properties of animal or human liver samples.
Uehara (1995) performed uniaxial tension tests on dog-bone shaped samples of porcine liver parenchyma at loading rates of 5, 20, 50, 200, and 500 mm/min [54]. The liver parenchyma samples were obtained from 7-month old pigs within 6 h of death (never frozen). Tests were performed at 21 °C and specimen hydration was maintained with Ringer’s solution. It was reported that the failure stress and modulus increased with increased loading rate, while the extension ratio decreased with increased loading rate. The average failure stress, average modulus, and average extension ratio ranged from 126 to 205 kPa, 0.68 to 1.19 MPa, and 1.29 to 1.26 mm/mm between loading rates of 5 and 500 mm/min, respectively.
Hollenstein et al. (2006) performed uniaxial tension tests on the capsule of one bovine liver, which was tested within 8 h of death [55]. The liver was chilled with wet ice and wrapped in physiologic saline soaked cloth between the time of procurement and sample preparation. The capsule samples were separated from the underlying parenchyma by gently peeling the capsule off of the parenchyma using the fingers and surgical forceps. It was reported that the nominal failure stress for the bovine liver capsule was 9.2 MPa and nominal failure strain was 35.6 %.
Santago et al. (2009) evaluated the effect of temperature on the tensile material properties of bovine liver parenchyma at a strain rate of 0.07 s−1 [56]. Uniaxial tensile tests were conducted on fresh bovine liver parenchyma using dog-bone shaped samples. For this study, one bovine liver was received within 24 h of death. The organ was sealed in a plastic bag and chilled with wet ice between the time of procurement and arrival. Samples were allowed to hang under their own weight, i.e. 1 g of tension, during the clamping process to ensure that all samples had a minimal but consistent initial preload. The state of strain under this minimal preload was used to define the point of zero strain. It was reported that there were no statistically significant differences in failure stress or strain between specimens tested at 75 °F versus those tested at 98 °F. The average 2nd Piola Kirchhoff failure stress and Green-Lagrange failure strain for all samples in this study was 52.5 kPa and 0.25 strain respectively.
In a second study, Santago et al. (2009) evaluated the effect of freezing on the tensile material properties of bovine liver parenchyma at a strain rate of 0.07 s−1 [57]. As with the previous study [56], uniaxial tensile tests were performed on bovine liver parenchyma using dog-bone shaped samples. For this study, one bovine liver was received within 24 h of death. The organ was sealed in a plastic bag and chilled with wet ice between the time of procurement and arrival. Upon arrival, the liver was sectioned into two equal halves. One half was prepared and tested immediately after arrival. The second half was frozen for 26 days and then tested after thawing. The frozen half of the liver was thawed in a saline bath at 75 °F for 12 h. Again, specimens were allowed to hang under their own weight, i.e. 1 g of tension, during the clamping process to ensure that all specimens had a minimal but consistent initial preload. The state of strain under this minimal preload was used to define the point of zero strain. It was reported that freezing resulted in a significant reduction in Green-Lagrange failure strain, but there were not statistically significant differences in the 2nd Piola Kirchhoff failure stress.
Brunon et al. (2010) conducted quasi-static (0.001–0.01 s−1) tensile failure tests on both fresh and previously frozen porcine and human liver capsule samples, which had the underlying parenchyma attached [58]. The porcine livers were obtained from adult pigs within 4–5 days after death. Fresh porcine samples were tested immediately, while frozen samples were stored in a freezer (24 h to a few days) until testing. Fresh, non-frozen human liver samples were tested within 5 days of death. The storage time between death and testing for frozen human liver samples was not reported. The mean ultimate true failure stress and Green-Lagrange failure strain for all fresh human hepatic capsule-parenchyma specimens were found to be 1.85 MPa and 32.6 %, and the mean ultimate true failure stress and Green-Lagrange failure strain for all fresh porcine hepatic capsule-parenchyma specimens were found to be 2.03 MPa and 43.3 %. A comparison between human and porcine tissues showed that the capsule characteristics were similar between porcine and human. However, freezing was found to significantly affect the failure properties of porcine liver capsule but not human liver capsule.
Kemper et al. (2010) performed uniaxial tension tests on dog-bone shaped samples of human liver parenchyma at loading rates of 0.01, 0.1, 1.0, and 10.0 s−1 (Fig. 14.5) [59]. Each organ was procured within 24 h of death, received within 36 h of death, and tested within 48 h of death to minimize the adverse effects of tissue degradation. The organs were immersed in Dulbecco’s Modified Eagle Medium (DMEM), a tissue culture medium, and chilled with wet ice between the time of procurement and specimen preparation. Immediately prior to mounting the specimens on the experimental setup, the specimens were immersed in a bath of DMEM heated to 37 °C. In this study, specimens were allowed to hang under their own weight, i.e. 1 g of tension, during the clamping process to ensure that all specimens had a minimal but consistent initial preload. The state of strain under this minimal preload was used to define the point of zero strain. The results of this study showed that the response of human liver parenchyma is both non-linear and rate dependent in tensile loading (Fig. 14.6). Specifically, failure stress significantly increased with increased loading rate, while failure strain significantly decreased with increased loading rate. The average 2nd Piola Kirchhoff stresses for loading rates of 0.01, 0.1, 1, and 10 s−1 were reported to be 40.2, 46.8, 52.6 and 61.0 kPa, respectively. The average Green-Lagrange failure strains for loading rates of 0.01, 0.1, 1, and 10 s−1 were reported to be 0.34, 0.32, 0.30, and 0.24 strain, respectively.
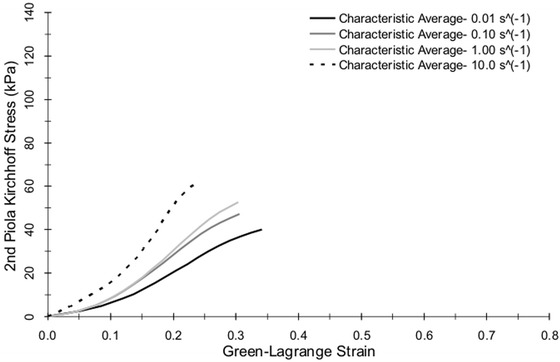

Fig. 14.6
Characteristic averages for tensile material response of human liver parenchyma dog-bone samples presented by Kemper et al. [59]
Kemper et al. [59] also compared the results of the human liver parenchyma material response to the material response of bovine and porcine liver parenchyma presented by previous authors [54, 56, 57]. The comparison showed that the tensile failure stress of porcine liver reported by Uehara [54] was significantly larger than the tensile failure stress of human liver. In contrast, the comparison also showed that neither the tensile failure stress nor the tensile failure strain of human liver was significantly different from that of bovine liver reported by Santago et al. [56, 57]. It was noted that the differences between human, porcine, and bovine liver parenchyma could potentially be attributed to the differences in the microstructure of the liver parenchyma between each of these mammalian species. Specifically, the delineation of adjacent hepatic lobules in porcine liver parenchyma is different than that of human and bovine liver parenchyma. The hepatic lobules are a roughly hexagonal arrangement of hepatocytes radiating from a central vein to portal triads located at the vertices of the lobule. Each of the portal triads contains a bile duct, proper hepatic artery, and hepatic portal vein. In porcine liver, the lobules are easily recognized in histology sections because the portal triads are connected by relatively thick layers of collagenous septum [7, 60, 61]. Conversely, adjacent lobules are indistinctively separated from one another in human and bovine liver since there is no interlobular collagenous septum [60, 62, 63]. This accounts for the tougher nature of porcine liver compared to bovine liver [63]. Although collagen content was not quantified by Kemper et al. [59], previous studies have shown that the stiffness of the human liver increases with respect to the degree of liver fibrosis [64]. Since liver fibrosis is defined as the excessive accumulation of extracellular matrix proteins, primarily collagen, the stiffness of the liver can be considered proportional to the collagen content. Therefore, the considerably higher collagen content present in porcine liver compared to human and bovine liver could explain the significantly higher failure stress observed in porcine liver.
14.4.3 The Liver in Compression
There have been a number of studies that have investigated the compressive material properties of liver by performing compression tests on isolated samples of liver parenchyma. The majority of these studies have been limited to sub-failure loading on animal tissue [64–68]. However, this summary is limited to studies that have reported compressive failure properties of animal or human liver parenchyma.
Tamura et al. (2002) performed uniaxial unconfined compression tests on rectangular, previously frozen (~24 h), porcine liver parenchyma samples tested at loading rates of 0.005, 0.05, and 0.5 s−1 [69]. A series of pre-conditioning compression tests were performed on fresh and previously frozen porcine liver specimens from which it was determined that freezing had no considerable effects on the mechanical response of the tissue. However, it is possible that damage to the tissue during the pre-conditioning may have masked any potential changes in the compressive response resulting from the freezing process. The specimens were tested while immersed in a constant temperature (36 °C) bath of physiologic saline. The results of the study showed that the response of porcine liver parenchyma is both non-linear and rate dependent. Specifically, the average peak Cauchy stress increased with increased loading rate: 123.4, 135.2, and 162.5 kPa, respectively. The peak nominal strain, however, was not found to vary considerably with respect to loading rate: 0.432, 0.420, and 0.438 strain, respectively. It should be noted that the initial specimen height, i.e. point of zero strain, was defined as the height that corresponded to an arbitrary load of 0.6 N, and Cauchy stress was calculated by assuming incompressibility, i.e. constant volume.
Pervin et al. (2011) performed uniaxial unconfined compression tests on fresh “ring” shaped bovine liver parenchyma samples at loading rates between 0.01 and 3,000 s−1 [70]. The specimens were tested at room temperature within 3 h of death. Intermediate (1, 10, and 100 s−1) and quasi-static (0.01 and 0.1 s−1) unconfined compression experiments were performed using a hydraulically driven MTS. High strain rate (1,000, 2,000, and 3,000 s−1) experiments were conducted using a Kolsky bar modified for soft material characterization. The point of zero strain was defined based on the measured initial specimen height, but the area of each sample was assumed to be equal to the ideal specimen area. The results of this study showed that the response of bovine liver parenchyma is both non-linear and rate dependent. It was reported that the average peak engineering stress increased with increased loading rate from 0.01 to 100 s−1: 49.7, 62.3, 94.8, 117.2, and 131.1 kPa, respectively. The engineering peak strain (i.e. nominal strain) was not found to vary considerably with respect to loading rate until after 1.0 s−1: 0.60, 0.60, 0.60, 0.59 and 0.55 strain, respectively. It was reported also that there were no considerable differences between samples oriented parallel to the exterior surface of the liver and samples oriented orthogonal to the exterior surface of the liver at intermediate and quasi-static loading rates, indicating that the bovine liver parenchyma is isotropic.
Kemper et al. (2013) performed uniaxial unconfined compression tests on cylindrical samples of human liver parenchyma at loading rates of 0.01, 0.1, 1, and 10 s−1 [71]. Each organ was procured within 24 h of death, received within 36 h of death, and tested within 48 h of death to minimize the adverse effects of tissue degradation. The organs were immersed in DMEM and chilled with wet ice between the time of procurement and specimen preparation. Immediately prior to testing, the specimens were immersed in a bath of DMEM heated to 37 °C. The point of zero strain was defined based on the initial specimen height, which was quantified using pre-test pictures. True stress was calculated based on the area of each cylindrical specimen, quantified using pre-test pictures, and assuming incompressibility, i.e. constant volume. The results of this study showed that the response of human liver parenchyma is both non-linear and rate dependent. Specifically, failure stress significantly increased with increased loading rate, while failure strain significantly decreased with increased loading rate. The average true failure stresses for loading rates of 0.01, 0.1, 1, and 10 s−1 were reported to be 63.7, 75.9, 103.4 and 110.5 kPa, respectively. The average true failure strains for loading rates of 0.01, 0.1, 1, and 10 s−1 were reported to be 0.94, 0.73, 0.61, and 0.60 strain, respectively.
14.4.4 The Spleen in Tension
There have been only two studies to the authors’ knowledge that have investigated the tensile material properties of spleen by performing tension tests on isolated samples of spleen parenchyma or spleen capsule. The experimental methods and key results from these studies are summarized in the current section.
Uehara (1995) obtained spleen parenchyma samples from 7-month old pigs within 6 h of death (never frozen) [54]. The samples were tested in uniaxial tension using compression clamps at loading rates of 5, 20, 50, 200, and 500 mm/min. Tests were performed at 21 °C and specimen hydration was maintained with Ringer’s solution. It was reported that the failure stress and modulus increased with increased loading rate, while the extension ratio decreased with increased loading rate. The average failure stress, average modulus, and average extension ratio ranged from 60 to 101 kPa, 164 to 272 kPa, and 1.59 to 1.51 mm/mm between loading rates of 5 and 500 mm/min, respectively.
Kemper et al. (2012) performed uniaxial tension tests on dog-bone shaped samples of human spleen parenchyma and spleen capsule at loading rates of 0.01, 0.1, 1, and 10 s−1 [72]. The capsule specimens were prepared with the underlying parenchyma attached (referred to as capsule/parenchyma specimens). This was done because the intimate connections between the capsule and parenchyma, specifically the collagenous trabeculae which extend from the capsule into the parenchyma, made it virtually impossible to isolate the capsule from the underlying parenchyma without causing damage. Each organ was procured within 24 h of death, received within 36 h of death, and tested within 48 h of death to minimize the adverse effects of tissue degradation. The organs were immersed in DMEM and chilled with wet ice between the time of procurement and specimen preparation. Immediately prior to mounting the specimens on the experimental setup, the specimens were immersed in a bath of DMEM heated to 37 °C. Similar to a previous study by Kemper et al. [59], specimens were allowed to hang under their own weight, i.e. 1 g of tension, during the clamping process to ensure that all specimens had a minimal but consistent initial preload. The state of strain under this minimal preload was used to define the point of zero strain. The results of this study showed that the responses of human spleen parenchyma specimens and spleen capsule/parenchyma specimens are both non-linear and rate dependent in tensile loading. Specifically, the failure stress significantly increased while the failure strain significantly decreased with increased loading rate for both parenchyma and capsule/parenchyma specimens. In addition, the failure stress of the capsule/parenchyma specimens was found to be significantly greater than that of the parenchyma specimens, while the failure strain was not significantly different. For the spleen parenchyma specimens, the average 2nd Piola Kirchhoff failure stresses for loading rates of 0.01, 0.1, 1, and 10 s−1 were reported to be 16.5, 23.9, 31.5 and 33.8 kPa, respectively. The average Green-Lagrange failure strains for loading rates of 0.01, 0.1, 1, and 10 s−1 were reported to be 0.26, 0.21, 0.19, and 0.18 strain, respectively. For the spleen capsule/parenchyma specimens, the average 2nd Piola Kirchhoff failure stresses for loading rates of 0.01, 0.1, 1, and 10 s−1 were reported to be 43.6, 46.1, 68.4 and 65.3 kPa, respectively. The average Green-Lagrange failure strains for loading rates of 0.01, 0.1, 1, and 10 s−1 were reported to be 0.23, 0.20, 0.19, and 0.17 strain, respectively.
14.4.5 The Spleen in Compression
There have been two studies that have investigated the compressive material properties of spleen by performing compression tests on isolated samples of spleen parenchyma. The experimental methods and key results from both of these studies are summarized herein.
Tamura et al. (2002) performed uniaxial unconfined compression tests on rectangular, previously frozen (~24 h), porcine spleen parenchyma samples tested at loading rates of 0.005, 0.05, and 0.5 s−1. [69]. The specimens were tested while immersed in a constant temperature (36 °C) bath of physiologic saline. The results of this study showed that the response of porcine spleen parenchyma is both non-linear and rate dependent. Specifically, it was reported that the average peak Cauchy stress increased with increased loading rate: 107.5, 114.6, and 146.3 kPa, respectively. The peak nominal strain, however, was not found to vary considerably with respect to loading rate: 0.825, 0.809, and 0.834 strain, respectively. It should be noted that the initial specimen height, i.e. point of zero strain, was defined as the height that corresponded to an arbitrary load of 0.6 N, and Cauchy stress was calculated by assuming incompressibility, i.e. constant volume.
Kemper et al. (2011) performed uniaxial unconfined compression tests on cylindrical samples of human spleen parenchyma at loading rates of 0.01, 0.1, 1, and 10 s−1 (Fig. 14.7) [73]. Each organ was procured within 24 h of death, received within 36 h of death, and tested within 48 h of death to minimize the adverse effects of tissue degradation. The organs were immersed in DMEM and chilled with wet ice between the time of procurement and specimen preparation. Immediately prior to testing, the specimens were immersed in a bath of DMEM heated to 37 °C. The point of zero strain was defined based on the initial specimen height, which was quantified using pre-test pictures. Engineering stress was calculated based on the initial area of each cylindrical specimen, which was quantified using pre-test pictures. The results of this study showed that the response of human spleen parenchyma is both non-linear and rate dependent. Specifically, failure stress increased with increased loading rate, while failure strain decreased with increased loading rate. In order to be consistent with the data published by Kemper et al. (2013), the engineering stress and engineering strain data presented by Kemper et al. (2011) have been converted to true stress, assuming incompressibility, and true strain (Fig. 14.8). The average true failure stresses for loading rates of 0.01, 0.1, 1, and 10 s−1 were reported to be 20.7, 25.3, 32.3 and 39.1 kPa, respectively. The average true failure strains for loading rates of 0.01, 0.1, 1, and 10 s−1 were reported to be 0.52, 0.51, 0.48, and 0.46 strain, respectively.
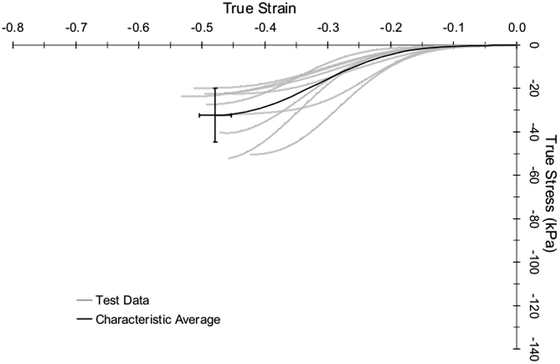

Fig. 14.8
Response of human spleen parenchyma in uniaxial compression at 1.0 s−1 presented by Kemper et al. [73]
14.5 Surrogate Testing
Historically, investigators have turned to human surrogates to learn about the impact and injury response of the abdomen. This includes animals, animal cadavers, and human cadavers (also known as post-mortem human surrogates, or PMHS). Human volunteers are enlisted only for quasi-static, sub-injury testing, or posture and position studies. Differences in size, shape, anatomy, and physiology complicate the translation of animal data to the human condition. Human cadaver testing is limited by post mortem changes. Cadavers have no muscle tone, no circulation, no respiration, and no physiological response or temperature. However, researchers attempt to make the cadaver respond more like the living by applying appropriate muscle/tendon tension when possible, or even inverting a specimen to counteract the effect of gravity, by perfusing and pressurizing the arterial and venous systems (sometimes with pulsatile flow), by ventilating the pulmonary system, and by heating the specimens. Regardless of the surrogate used, scaling the response data to the human is always an issue. Various scaling techniques have been proposed over the decades, but none has received universal acceptance, nor produced satisfying outcomes. This is particularly true when trying to assess pediatric response, for which there are precious few data.
Prior to the 1980s, little had been done to examine the response of the cadaver abdomen to impact. However, some animal testing had been conducted to investigate the response of specific organ systems as well as the abdomen as a whole [74–76]. Still today, the frontal crash regulatory dummy, the Hybrid III, does not have a biofidelic abdomen, and there are no abdominal injury criteria in the federal crash standards. Efforts began in the 1980s to develop improved crash dummies and parts thereof, such as the Advanced Anthropomorphic Test Device, or AATD, presented by Schneider et al. (1992), which fueled new interest in the response of the abdomen [77]. Several studies were conducted during that time, with some of the earliest being Rouhana et al. (1986), which studied the response of swine cadavers to seatbelt loading [78], and Cavanaugh et al. (1986), which studied the response of the cadaver abdomen to rigid-bar loading [79]. More recently, attention has focused on high-speed pretensioner loading under out-of-position conditions. Collectively, there is not an abundance of response data for the abdomen. And, some of the studies seem to provide conflicting results, although most of the discrepancies between datasets have been resolved [80]. Still, efforts continue to develop biofidelic abdominal inserts for crash dummies [81, 82], and corresponding injury metrics. This work extends in to the virtual world as well, with substantial effort being put toward the development of finite element representations of the human body.
Typically, researchers try to simulate interaction of a vehicle occupant with various aspects of the vehicle interior. Often, these interactions are simplified in an attempt to conduct more repeatable, and measurable, tests. Usually the tests are simplified in terms of occupant posture, seating system (or lack thereof), the type of interaction (e.g., rigid bars instead of steering rims), and the nature of the interaction (location and direction of applied load). For the abdomen, the specimen is usually positioned statically or even rigidly in a fixture and then impacted, avoiding the complications associated with sled testing. Studies have simulated interaction with the steering rim, steering column and dashboard components, restraint systems such as seatbelts and airbags, as well as passenger doors and armrests.
14.5.1 Rigid Impact
Steering rims are generally simulated using rigid bar indenters, both straight and curved. Other more blunt objects or surfaces within the occupant compartment, including steering column components, are often simulated using rigid disks of various diameters (152–305 mm). More penetrating interactions are simulated using smaller diameter probes (76 mm), which can be used for mapping the local, or regional, response of the abdomen to impact. These indenters and probes are typically fixed to heavy ballistic pendulums or linear impactors.
14.5.1.1 Frontal Impact
Stalnaker and Ulman (1985) compiled and analyzed data from three earlier studies [83]. These earlier studies were conducted by Beckman et al. (1971), Stalnaker et al. (1971), and Trollope et al. (1973), which all used rigid-bar and wedged-shaped impactors [74, 76, 84]. Tests were conducted using different primates ranging from 0.53 to 19.40 kg in free-back conditions and impact speeds ranging from 8.4 to 17.0 m/s. Three different impact locations were used, including the central regions of the upper, middle, and lower abdomen (mostly upper abdomen). All of the response data from the three different regions and six different impact surfaces was plotted on the same graph to define a single abdominal response corridor for 12.1 m/s. The force-deflection data were described in terms of a three-stage rise-plateau-rise response.
Cavanaugh et al. (1986) conducted abdominal impacts into 12 unembalmed cadavers using a 2.5-cm diameter, 38.1-cm long rigid bar attached to 31.5 and 63.5-kg linear impactors using impact speeds ranging from 4.9 to 13.0 m/s [79]. The bar was oriented with the long axis parallel to the width of the subject at the level of the third lumbar vertebra. Each subject was seated upright in a free-back condition, with the legs positioned straight out from the cadaver, with the torso held upright by straps under the arms that were released at the time of impactor contact. These tests were separated into two groups having average speeds of 6.1 +/− 1.5 m/s (five tests) and 10.4 +/− 1.1 m/s (seven tests). Four of the higher speed tests employed the 63.5-kg impactor. Importantly, abdominal response was found to be proportional to both impact speed and impactor mass, suggesting rate sensitivity of the response.
Viano et al. (1989) conducted impacts into unembalmed, repressurized free-back human cadavers using a 15.2-cm diameter rigid disk attached to a 23.4-kg pendulum and three speed ranges, averaging 4.5, 6.7, and 9.4 m/s [85]. Tests were performed at angles of 60° to the right or left of the midline through the center of gravity of the specimen, with the center of the impactor at 7.5 cm below the xiphoid process, covering approximately ribs 6–10. Deflection data was obtained by analysis of high-speed film. The subjects were suspended upright, with hands and arms overhead. The loading response showed a force plateau following the initial stiffness response, and the unloading response suggested action of restorative forces, which contrasts the sudden unloading reported by Cavanaugh et al. [79].
Nusholtz and Kaiker (1994) conducted impacts at the level of the second lumbar vertebra using six unembalmed, repressurized human cadavers [86]. The cadavers were positioned in a free-back seated posture with the knees bent and the legs hanging down. The impact apparatus consisted of an angled semicircular rigid-tube attached to an 18-kg pendulum, simulating the lower rim of a steering wheel. The bottom of the rim impacted equidistant from the inferior aspect of the tenth ribs and the iliac crests. A harness that was released at contact was used to keep the cadavers upright. Tests were conducted at nominal impact speeds of 6 and 10 m/s, varying from 3.9 to 10.8 m/s. In contrast to the Cavanaugh results, no rate sensitivity in the loading response was found.
Yoganandan et al. (1996) performed oblique lateral pendulum impacts similar to those of Viano et al. [85] on the right lower thorax (upper abdomen) of five human cadavers [87]. A 23.5-kg impactor with a 15.2-cm diameter, Ensolite-padded impact face was used, having velocity of 4.3 m/s. A chestband was used to monitor the contour of the cadavers during the impact. Force-deflection curves were presented, but the responses were not normalized for mass, which ranged from 56 to 82 kg. Two of the five curves showed large variability. The reasons for this variability were not addressed, but might include rotation of the test subjects, as there is no mention of an effort to strike through the center of mass of the subjects to prevent rotation, as was done by Viano et al. [85].
Of the aforementioned rigid impact studies, a number of them warranted further analysis to get a better understanding of the reasons for some of the seeming discrepancies between the reported responses in terms of shape and rate sensitivity. In 2001, Hardy, Rouhana, and Schneider [80] reanalyzed the existing body of rigid-bar abdominal-impact data by first digitizing the published results of the investigations of Stalnaker and Ulman [83], Cavanaugh et al. [79], Viano et al. [85], and Nusholtz and Kaiker [86]. For simplicity, only eight points were taken from each curve for this analysis. The Nusholtz cadaver data were split into high- and low-speed corridors, and equal-stress/equal-velocity scaling was applied. The Viano cadaver data were averaged within the 6.7- and 9.4-m/s ranges to yield two mean curves. Upper abdomen tests were eliminated from the Stalnaker primate data, and of the remaining tests, those conducted at 10 m/s, +/− 1.5 m/s were selected for analysis (seven tests). Again, equal-stress/equal-velocity scaling was applied. The data were averaged across all species to obtain the “human” response. The prescribed three loading stages were generated using 9.6 % and 27 % compression break points, and the data were normalized to an abdominal depth of 289 mm. Velocity scaling was used to generate a 6-m/s curve.
Because the Cavanaugh rigid-bar data showed a rate-dependent response, the Cavanaugh corridors were plotted for comparison to other reanalyzed datasets. The comparisons were simplified by digitizing five-to-eight points for each boundary of the corridors, which were piecewise continuous linear approximations of the continuous data. Similarly, for ease of comparison, the corridors were truncated at 9 kN for the 10-m/s corridor and 140 mm for the 6-m/s corridor. These comparisons are shown in Figs. 14.9 and 14.10. Although the various datasets show differences in curve characteristics, there is overall agreement in terms of stiffness for the 10-m/s corridor. For the 6-m/s corridor, there are differences in terms of curve shape and loading rate. This exercise suggests that the apparent rate effects, whether of a viscous or mass-recruitment nature, depend on the relative impactor-to-subject mass ratio, with higher ratios resulting in more rate sensitivity. The loading-phase characteristics depend on impactor shape, with larger relative surface area (distributed interface) resulting in a steeper rise to a plateau, and narrower bars (focused loading) resulting in a more gradual rise with no plateau. The degree of hysteresis during unloading is related to impact location and the nature of the impactor. Larger loading surfaces that result in contact with the ribcage, which produces restorative forces, are associated with less hysteresis than impacts that do not engage the ribcage. Focused loading that does not involve the ribcage generally results in a rapid drop in force for little reduction in penetration during the unloading phase of an abdominal impact.
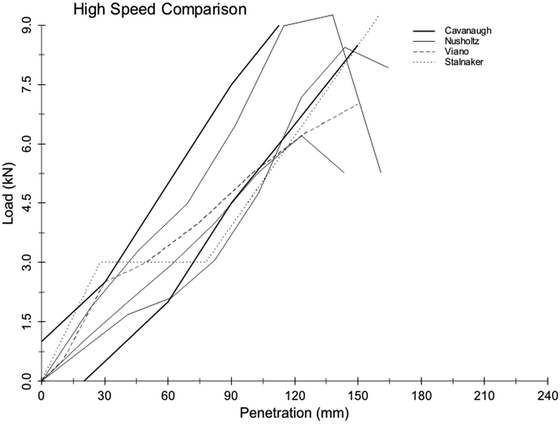
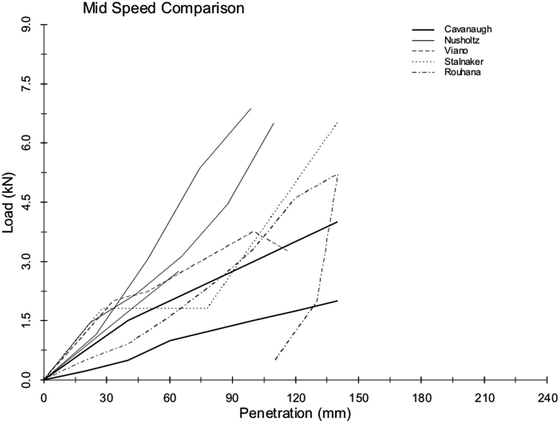

Fig. 14.9
Reanalysis of existing high-speed data (10 m/s) rigid-bar abdominal impact data showing reasonable agreement between studies [80] (Reprinted with permission from the Stapp Association)

Fig. 14.10
Reanalysis of existing mid-speed data (6 m/s) showing differences in stiffness between the various studies [80] (Reprinted with permission from the Stapp Association)
After reanalyzing the available rigid-bar data and forming theories to explain the differences observed between datasets, Hardy et al. [80] conducted new testing of the cadaver abdomen in an effort to support their theories. In addition, a goal of their research was to examine the response of the human cadaver abdomen to various types of frontal impact. This included rigid-bar, seatbelt, and close-proximity airbag loading. Each of these loading modes is described in subsequent sections herein, beginning with the rigid-bar tests.
Hardy et al. [80] conducted both free- and fixed-back impacts of the cadaver abdomen, in two regions. A 48-kg ballistic pendulum was fitted with a 2.5-cm diameter, 46-cm long, rigid bar. The 48-kg mass was used because it was the median of the masses used by Cavanaugh and it was substantially larger than the effective mass of a typical abdomen. Each cadaver was positioned in a seated, upright, free-back posture with the legs positioned forward on a curved plastic skid, with the hands positioned in front of and above the head, as shown in Fig. 14.11. Accelerometer blocks were attached to T11 and L3, as were dual-marker target masts to facilitate kinematics analysis using high-speed (1 kfps) film. Prior to impact, the lungs of each cadaver were expanded using compressed air. Heated, 13.8-kPa, normal saline was used to perfuse the arterial and venous systems. Each cadaver was suspended prior to impact, and released at the moment of impact so as to be unconstrained during impact. A cargo net caught the cadavers after impact and prevented additional damage.


Fig. 14.11
The free-back rigid-bar impact apparatus and configuration used by Hardy et al. (2001) to investigate the response of the PMHS abdomen [80] (Reprinted with permission from the Stapp Association)
Nine tests were conducted using nine cadavers with average age, stature, and mass of 78 years, 170 cm, and 68 kg respectively. Six tests were conducted at the mid abdominal level (L3), with three of these in the 6-m/s range (6.3 m/s average) and three in the 9-m/s range (9.2 m/s average). Three additional tests were conducted at the upper-abdominal level, with two of these in the 6-m/s range and one in the 9-m/s range. For these tests, the rigid bar made initial contact with the epigastric region, approximately at the level of T11.
A fixed-back condition was also investigated. This configuration was designed to eliminate the motion of the spine and the effects of whole-body mass. This was in large part because the study was conducted in support of a new dummy abdomen that Rouhana et al. (2001) were developing [81]. Therefore, it was desired to investigate the role of spinal flexion and cadaver mass on abdominal response. A single large male cadaver (175 cm, 88 kg, 80 years) was used for all fixed-back tests. The specimen was held in a seated posture, fastened to a rigid seat back using “u” clamps installed around the spine from behind. To avoid damage to the specimen during repeated testing, the pendulum motion was arrested after 200 mm of abdominal penetration. Steel cables were used to tether the pendulum to an energy-absorption apparatus that consisted of steel rods. As tension developed in the cables, the rods were bent, which dissipated excess energy in the system. This specimen was impacted seven times using three different speed ranges: 3, 6, and 9-m/s. The order of the tests was designed to minimize changes in the response due to previous testing, as well as to disclose the changes that occurred.
This new set of free-back rigid-bar tests corroborated the Cavanaugh results, and reinforced the assertion that the response of the abdomen is rate sensitive. However, the observed rate sensitivity could be due to either mass recruitment or tissue viscosity, or both. The 48-kg impactor was important to obtaining this response. This is because the response of a rate-sensitive system is affected by the magnitude and rate of energy transfer, which are significantly influenced by the relationship between the effective mass of the system and the mass of the striking object.
Hardy et al. [80] also developed new response corridors (Figs. 14.12 and 14.13). The average dynamic stiffness of the mid-speed tests was 27 kN/m compared to 21 kN/m for Cavanaugh. The average dynamic stiffness of the high-speed tests was 63 kN/m compared to 70 kN/m for Cavanaugh. Differences in impact speed could account for the slight differences in stiffness between the results for the two studies. The upper abdomen tests also showed greater initial stiffness in both speed ranges (6 and 9 m/s) than did the mid abdomen tests. Further, there was less hysteresis in the upper abdomen response. In keeping with the original theory developed from examining existing data, these findings are thought to be due to greater involvement of the ribcage.

Fig. 14.12
The low-speed (6-m/s), midabdomen, rigid-bar impact response corridor developed by Hardy et al. (2001) for PMHS abdomen loading [80] (Reprinted with permission from the Stapp Association)

Fig. 14.13
The high-speed (9-m/s), midabdomen, rigid-bar impact response corridor developed by Hardy et al. (2001) for PMHS abdomen loading [80] (Reprinted with permission from the Stapp Association)
The loading responses to rigid-bar testing of the upper abdomen (high speed) and the fixed-back tests were similar to the responses reported by Stalnaker, who characterized the loading curve as a steep rise, followed by a gradual slope or plateau, and then another steep rise. Greater rib involvement, as well as a greater degree of bottoming out against the spine, could have contributed to this rise-plateau-rise character. Melvin et al. (1988) suggested that for this type of response, the initial rise and subsequent plateau could be attributed to viscous characteristics, followed by inertial loading and tissue compression effects [88].
The fixed-back tests further also demonstrated the rate-dependent nature of the abdomen. The load-penetration responses of the fixed-back tests matched free-back results initially, but dropped below the corridors later in the impact. For the free-back tests, the reaction loads measured at the pendulum result from compressing abdominal tissue and accelerating the body. For the fixed-back tests, the reaction loads result from compressing abdominal tissue only. It is assumed that the whole-body motion of a free-back specimen would begin at a force level below that at which the fixed-back curves begin to fall below the corridors. Elimination of the inertial component of whole-body motion (i.e., removing a large mass-times-acceleration component) is likely the reason for the load-penetration responses for fixed-back tests falling below the corridors for free-back tests, in the absence of large compressive loads resulting from crushing tissues against the spine.
Howes et al. (2012) conducted tests using four PMHS that were subjected to four different fixed-back loading conditions [89]. The primary focus of this study was to quantify internal organ kinematics in blunt impacts to the thorax and abdomen. The secondary focus of this study was to gain an understanding of the structural interactions of the abdominal contents during impact to better understand injury mechanisms in motor vehicle crashes. High-speed biplane x-ray was used to image the motion of the thoracoabdominal contents in three dimensions. The quantification of relative internal organ kinematics during blunt impact can be used to identify potential crash-induced injury mechanisms for further investigation using finite element models and isolated experiments. For the abdomen (three cadavers), the loading modes consisted of a driver-side shoulder belt applied at a target speed of 3.0 m/s, a 114-mm diameter, 32.2-kg cylindrical probe impactor applied to the abdomen at 3.0 and 4.0 m/s without engaging the thorax, and a 25.4-mm diameter, 32.6-kg rigid-bar impact at 6.7 m/s centered at the mid-umbilicus. The two cadavers impacted in the abdominal region were subjected to multiple impacts of increasing severity. The two initial abdominal impacts were non-injurious, with the final test for each cadaver designed as a destructive test to induce a considerable amount of marker excursion for the instrumented organs. Tests were conducted in inverted, fixed-back configurations, as shown in Fig. 14.14. Tests were conducted using an inverted posture to adjust the initial position of the abdominal organs to obtain a more anatomically correct model [90], to counteract the effect of gravity.
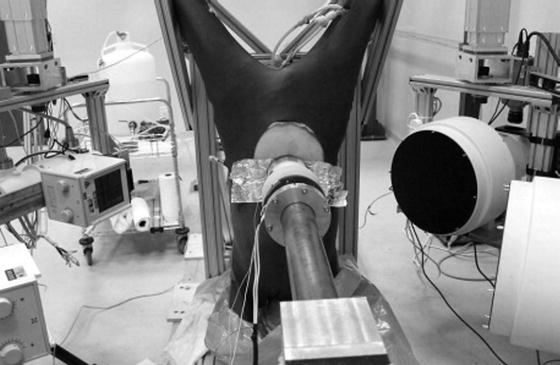

Fig. 14.14
The inverted cadaver position used by Howes et al. (2012) to investigate the response of the PMHS abdomen to probe impacts visualized by high-speed x-ray [89] (Reprinted with permission from the Stapp Association)
The probe impacts resulted in anatomically cranial and posterior marker trajectories. The diaphragm moved in a circular pattern with a slight anterior-superior trajectory initially, followed by an arc in the left posterior to right anterior direction. The liver moved in a distinct arc to the right before continuing posterior and inferior from the initial position. The right lobe of the liver showed greater excursion than the left lobe, but the patterns were similar. Motion of the stomach and mesentery occurred in less defined patterns, first shifting superior and anterior before arcing toward the left, inferior, posterior region. The marker motion for an exemplar 3-m/s probe impact as measured by high-speed biplane x-ray is shown for the sagittal and transverse planes in Figs. 14.15 and 14.16, respectively.
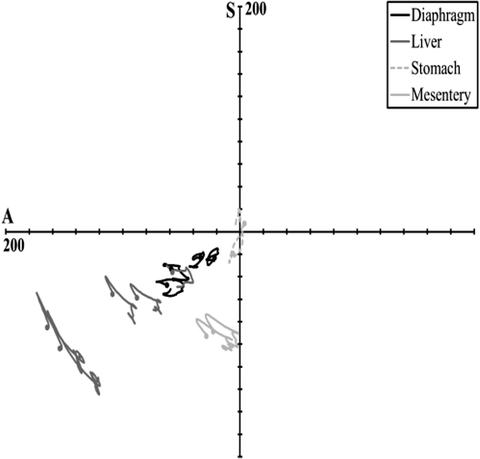
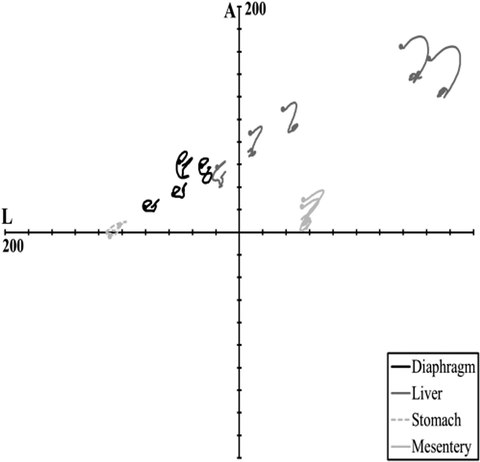

Fig. 14.15
A sagittal plane perspective of marker/organ motion for a 3-m/s probe impact of the PMHS abdomen from Howes et al. (2012), measured using high-speed biplane x-ray [89] (Reprinted with permission from the Stapp Association)

Fig. 14.16
A transverse plane perspective of marker/organ motion for a 3-m/s probe impact of the PMHS abdomen from Howes et al. (2012), measured using high-speed biplane x-ray [89] (Reprinted with permission from the Stapp Association)
Building upon the notion that inversion of a cadaver can place the thoracoabdominal contents closer to the in-vivo condition, Howes et al. (2013) investigated this hypothesis directly [91]. Biplane x-ray was used to image two cadavers in upright and inverted postures, and the three-dimensional variation in the relative abdominal organ position was quantified. The abdominal organs were instrumented using radiopaque markers. The specimens were ventilated and perfused, and residual air was removed from the abdominal cavity. Intuitive changes in organ position were observed due to the effect of gravity. When upright, the superior-inferior separation between the diaphragm and liver markers ranged from 95 to 169 mm, but when inverted, the organs shifted cranially and the separation fell to within 66 to 81 mm. These data were scaled and compared to the Global Human Body Models Consortium (GHBMC) model geometry and to the positional MRI data of Beillas et al. (2009) from nine human subjects in seated postures [92]. The overall shapes and relative positions of the inverted cadaver organs were deemed to compare better to both the human subjects and model geometry. These results reinforce the idea that interpretation of cadaver test results and comparison to finite element simulations is not straightforward. The ability of a model to have the same mechanical response and to predict the same injuries as observed during tests for which the abdominal viscera are in substantially different locations and orientations is very limited. This phenomenon can explain difficulties in using historical cadaver testing data for model development and validation. It is also an important consideration for investigators designing cadaver tests in the future: the mechanical response and injury patterns will be potentially more accurate for the inverted case, and the results will be more directly comparable to modeling efforts.
14.5.1.2 Side Impact
Walfisch et al. (1980) dropped unembalmed human cadaver subjects from one and two-meter heights to examine lateral impact response and injury [93]. The surface impacted was either a rigid or deformable simulated armrest that was struck by the right side of the subject at the level of the ninth rib. Contact speeds were 4.5 m/s for the 1 m drop, and 6.3 m/s for the 2 m drop. Deflection data was determined by film analysis, where deflection was defined as intrusion of the armrest relative to the spine, not the opposite side of the subject. Force data were obtained from load cells located beneath the simulated armrest. The data for the rigid armrest were normalized using the method proposed by Mertz [94] for lateral impact by the International Standards Organization [95].
Cavanaugh et al. (1996) reported the results of 16 side impact sled tests using a rigid or padded flat wall or a simulated armrest [96]. The setup was similar to that used at the University of Heidelberg [97] by Kallieris et al. (1981) in the tests which served as the basis for the Thoracic Trauma Index (TTI). The tests used a deceleration sled traveling 6.7 or 8.9 m/s that impacted a hydraulic snubber. During deceleration, the cadaver slid across a low friction surface such that the left side of the subject struck a rigid or padded wall that was instrumented with load cells. In some tests, the subject impacted a simulated armrest made of either soft or stiff paper honeycomb, nominally 69 or 138 kPa crush strength.
14.5.2 Restraint Testing
Various types of occupant restraints have been tested using animals and cadavers. These include primary seatbelt systems and supplemental passenger airbags. Both lap-belt only and lap-shoulder configurations have been tested, with some attention paid to load limiting, and considerable attention given to pretensioner loading. The 4-point restraint system developed by Rouhana et al. (2003) remains to be tested with respect to abdomen interaction [98]. Given repeatability and measurement issues, the impact response to airbags has been investigated using a surrogate airbag as well.
14.5.2.1 Seatbelt Tests
Although part of the recent effort of Howes [89] involved loading of the thorax and abdomen using a driver shoulder belt, the focus of that study was not interaction of the seatbelt with the abdomen. Several earlier studies have focused on this interaction using animal, animal cadaver, and human cadaver experimental models. Commonly, swine and swine cadaver models have been selected to investigate abdominal response to seatbelt loading. In particular, Rouhana et al. (1986) conducted 15 impacts on swine cadavers using a controlled-stroke MTS machine [78]. Also, Miller (1989) conducted 25 dynamic belt-loading tests into the abdomens of anesthetized swine [99]. These belt-loading tests were performed using a haversine displacement-time function with peak speeds near 3.7 and 6.3 m/s (“low” and “high” speeds). The animals were placed in a supine position in a V-shaped back support. An arch-shaped yoke was used to drive seatbelt webbing in to the abdomen at the level of the fourth lumbar vertebra. The load-penetration responses were characterized by a slightly convex ramp averaging 30 kN/m, followed by an essentially vertical unloading. Peak compression ranged from 6 % to 67 %. The swine cadaver and anesthetized swine data were compared to examine the scaling possibilities between human cadavers and living humans. Normalization was performed by Rouhana et al. (1989 and 1990) using equal-stress/equal-velocity scaling to account for differences between subject mass and anteroposterior dimension, and the data were separated in to low and high-speed corridors [100, 101]. These corridors were scaled by van Ratingen et al. (1997) to develop biofidelity seatbelt loading performance targets for the Q3, 3-year old child dummy [102]. These swine data were used by Rouhana to design the frangible abdomen for the Hybrid III dummy.
Hardy et al. (2001) conducted six seatbelt tests using three cadavers and a peak-loading rate of 3 m/s [80]. The cadavers were loaded about the mid abdominal region, approximately at the level of the umbilicus, as shown in Fig. 14.17. The webbing was placed flat against the anterior surface of the abdomen, and fastened to a pneumatic driving mechanism behind the cadaver. The geometry of the belt was designed to optimize belt/abdomen interaction, and to minimize “roping” of the belt. The seatbelt webbing was routed straight back from the sides of the cadaver. Peak loading was roughly 3 m/s using an approximately haversine penetration speed-time history. A seatbelt loading response corridor was generated for these data (Fig. 14.18). The initial stiffness was found to be 120 kN/m which is four times greater than the results of Miller [99], and probably results from differences in the way the tests were conducted as well as differences in the test subjects. Miller used a yoke device that resulted in the ventral-most portion of the abdomen being loaded initially, with an increasing section of abdomen being loaded as the yoke displacement progressed. The contact area increased as the penetration increased, but the sides of the abdomen were not particularly constrained. Hardy et al. [80] wrapped the webbing around the anterior and lateral aspects of cadaver abdomens. The contact area was quite large from the beginning of each test, and resulted in a more distributed and consistent application of load around the circumference of the abdomen, with the webbing restricting lateral motion of the sides of the abdomen. Both of these factors are thought to be responsible for the higher stiffness values found by Hardy.


Fig. 14.17




The free-back seatbelt loading apparatus and configuration used by Hardy et al. (2001) to investigate the response of the PMHS abdomen [80] (Reprinted with permission from the Stapp Association)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree