Chapter 99 Hypericum perforatum (St. John’s Wort)
Hypericum perforatum (family: Hypericaceae)
Common names: St. John’s wort, Klamath weed, hypericum
 General Description
General Description
Likely the most well-studied botanical of all time, St. John’s wort (Hypericum perforatum) is a shrubby perennial plant with numerous bright yellow flowers. It is commonly found in dry, gravelly soils, fields, and sunny places. St. John’s wort is native to many parts of the world, including Europe, Asia, and the United States. It grows especially well in northern California and southern Oregon.1
The plant is glabrous throughout, green or sometimes glaucescent; the stems are erect, branched at the top, and 30 to 100 cm long. The leaves are (1) oval or elliptic; (2) oblong-ovate or rather narrow, oblong-linear, subobtuse, and flat; or (3) more or less revolute—marginated with numerous pellucid and a few black granular dots. The yellow flowers are numerous, forming a broad, paniculate, almost corymbose inflorescence, 7 to 11 cm long and 5 to 11 cm broad. The lanceolate bracts are 0.5 cm long and acute. The calyx is deeply parted, 5 mm long, and about two to three times shorter than the corolla. The sepals are lanceolate or narrow lanceolate 4 to 5 mm long, 1 mm broad, as long as the ovary, acute or acuminate, sparingly furnished with black oval dots, with a smooth or sparsely toothed margin. The petals are oblong to oblong-elliptic, 1.2 to 1.5 cm long and 0.5 to 0.6 cm broad, with or without numerous black granular dots and lines on the margin in the upper part, whereas the surface is full of yellow glandular dots, thin lines, and stripes. The three-bundled stamens are numerous; the ovary is ovoid, 3 to 5 mm long. The seed is 1 mm long, cylindric, brown, and minutely pitted longitudinally.1
The whole plant is used medicinally. Harvesting time is generally July through August. The plant must be dried immediately to prevent degradation of active principles.1
 Chemical Composition
Chemical Composition
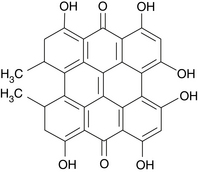
The major compounds of interest are hypericin (Figure 99-1) and pseudohypericin. These compounds are typically found in low concentrations, ranging from 0.0095% to 0.466% in the leaves and as much as 0.24% in the flowers.1
More recently, researchers have been interested in the other chemical constituents (especially the various flavonoids and xanthones). The interest in these other components stems largely from pharmacologic studies with commercially available extracts demonstrating effects and benefits beyond hypericin and pseudohypericin. For example, amentoflavone was shown to bind to benzodiazepine receptors and to act as a modulator of γ-aminobutyric acid, which may be a major anxiolytic mechanism.2 The active components include1,3:
 History and Folk Use
History and Folk Use
More than 27 double-blind randomized trials, involving more than 2200 patients with mild to moderately severe depression, showed that St. John’s wort extracts standardized for hypericin had excellent results in the treatment in depression, with far fewer side effects than standard antidepressant medications.4 For patients with preexisting conductive heart dysfunction or elderly patients, high-dose hypericum extract is safer with regard to cardiac function than tricyclic antidepressants.5 It is no coincidence that St. John’s wort extracts became among the most widely used antidepressants in Germany, with a market share of more than 25% in 1997.6,7
 Pharmacology
Pharmacology
St. John’s wort extracts (primarily of the flowering tops) have shown a wide variety of effects in experimental and clinical studies. Some of the activities demonstrated include the following1,3:
Antidepressant Activity
As stated earlier, initial studies indicated that St. John’s wort extract’s antidepressant action was based on the ability of crude hypericin preparations to inhibit both types A and B MAO.8,9 As a result of this inhibition, there is an increase in the level of neurotransmitters within the brain that maintain normal mood and emotional stability, including serotonin, catecholamines, and dopamine. These preliminary results identified hypericin as the supposed active constituent. However, later chemical analysis of these crude hypericin preparations identified the active content as being as much as 20% of St. John’s wort’s other constituents, with flavonoids being the most important.1 In other words, it is unknown to what extent hypericin or the flavonoids individually contribute to any MAO inhibition.
A study was conducted to better understand the influence of hypericin, hypericum total extract, and hypericum fractions on the activity of MAO and COMT.10 An inhibition of MAO was shown using the following concentrations:
A COMT inhibition could not be shown for hypericin, with hypericum extract to 10−4 mol/L and with two extract fractions also up to 10−4 mol/L. The MAO inhibiting fraction contained hypericins, as well as flavonols, the COMT-inhibition fraction being mainly flavonols and xanthones. The key result from this study, as well as in another in vitro/ex vivo study, was the demonstration that the concentrations of inhibition shown, particularly with regard to the inhibition of MAO activity, were likely insufficient to explain the clinically proven antidepressive effect of St. John’s wort extract.10,11 Therefore, additional mechanisms are likely responsible for these clinical benefits.
At least three other mechanisms have been proposed: modulation of interleukin-6 (IL-6) activity, inhibition of the reuptake of serotonin, and agonist action of σ-receptors. The modulating effect of St. John’s wort extract on IL-6 is the most interesting, as it proposes a mechanism by which St. John’s wort interacts with the link between the immune system and mood. The immune system and the nervous system share many common biochemical features and regulatory interactions. The cytokine, IL-6, is heavily involved in the communication between cells within and outside the immune system. With regard to the nervous system, IL-6 is known to modulate hypothalamic-pituitary-end organ axes, especially the hypothalamic-pituitary-adrenal axis. The hypothesis is that an elevation in IL-6 results in activation of the hypothalamic-pituitary-adrenal axis, leading to elevations in corticotropin-releasing hormone and other adrenal regulatory hormones—hallmark features in depression. St. John’s wort extract showed an ability to reduce IL-6 levels; therefore, this action may explain the clinical effectiveness of St. John’s wort extract.12
Animal studies comparing the effects of hypericum in mice who produce normal IL-6 levels versus IL-6 knockout mice (those with no IL-6 expression), found higher levels of serotonin produced in the former group.13 An in vitro study demonstrating reduction of IL-6 levels involved taking blood samples from five healthy volunteers and four depressive patients.12 The release of IL-6, IL-1β, and tumor necrosis factor-α was measured quantitatively after an incubation time of 24 hours on microtiter plates. A massive suppression of IL-6 release was found for phytohemagglutinin-stimulated St. John’s wort extract. If these effects can be duplicated in vivo, it would provide a mechanism by which St. John’s wort extract modulates release of corticotropin-releasing hormone and, subsequently, mood.
St. John’s wort extract was also shown to inhibit the reuptake of serotonin in a similar fashion to drugs like fluoxetine (Prozac), paroxetine (Paxil), and sertraline (Zoloft). The study that demonstrated a 50% serotonin reuptake inhibition used the 0.3% hypericin content standardized extract at a concentration of 6.2 mg/mL and did not attempt to identify the active inhibitors.14 The authors of the study concluded that “the antidepressant activity of hypericum extract is due to inhibition of serotonin uptake by postsynaptic receptors.” However, results were conflicting with regard to the effect of acute doses of St. John’s wort extracts on the serotonergic system in rodents.15 A marked increase of both serotonin and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) was reported in rat brain cortex. Other researchers reported high 5-HIAA and serotonin levels in the mouse hypothalamus and hippocampus, but not in the cerebral cortex, where only 5-HIAA increased. However, other research found no significant changes in either serotonin or 5-HIAA after a schedule of treatment during the forced swimming test. One study of the rat locus coeruleus showed that systemic hyperforin induced a lasting and marked (100% to 200%) increase in the extracellular concentrations of serotonin, but not 5-HIAA.16
There is gathering information that St. John’s wort may affect function via σ-receptors. Sigma1-receptors were shown to play an important role in antidepressive effects because selective σ1-receptor agonists, as well as typical antidepressants, reduced the immobility time in the forced swimming and tail suspension tests.17 Research demonstrated that the pretreatment of rats with St. John’s wort extracts or hyperforin trimethoxybenzoate could block other ligand binding to σ-receptors. The antidepressant-like activity of hyperforin trimethoxybenzoate was completely antagonized by pretreating rats with BD 1047, a selective σ1 antagonist. These results, together with the previous observation that agonists at σ1 receptors are active in antidepressant models in rats, suggest that St. John’s wort’s antidepressant effects might be mediated in part by an indirect action on σ-receptors.15
A fascinating study evaluated the effect of both St. John’s wort and imipramine on gene transcription in the rat hypothalamus.6 The depressed patient’s hypothalamus is known to manifest changes associated with food intake, decreased libido, and abnormal circadian rhythms. This research found a significant correlation of six genes directly modulated by both these test compounds. The probability of this occurring by chance was 1.14 × 10−23. The functions of these specific genes include protein synthesis and degradation, cellular scaffolding and intracellular transport, mitochondrial and glycolytic energy metabolism, and cellular signaling through modulation of calcium-binding proteins. Although the implication of this research is not totally clear, the authors postulated that these modulated gene functions might be related to immune and inflammatory downregulation with neuronal protection from cellular injury.
Amentoflavone, one of the many flavone contents in St. John’s wort, was shown to cross the blood–brain barrier by passive diffusion.18 This might account for the ability of this botanical to travel into the brain and affect CNS gene transcription. Nevertheless, one point regarding the probable synergistic effects of the St. John’s wort constituents should be underscored: given the multitude of active components in this botanical medicine, it is likely that synergistic sites of action outside the CNS, in accordance with CNS effects, account for the complete and effective global actions of St. John’s wort.
Extracts of St. John’s wort were tested in various animal models designed to study its antidepressant effects. In these studies, St. John’s wort extract was found to enhance the exploratory activity of mice in a foreign environment, extend the narcotic sleeping time in a dose-dependent fashion, antagonize the effects of reserpine, and decrease aggressive behavior in socially isolated male mice.1,19 These activities are consistent with the expected effects of antidepressant compounds and appear to be the result of increased monoamine activity.
Antiviral Activity
In vitro studies showed that hypericin and pseudohypericin exhibit strong antiviral activity against herpes simplex virus I and II, as well as influenza types A and B, and vesicular stomatitis virus.20 These compounds also demonstrated remarkable antiviral activity against Epstein-Barr virus.21
Researchers from New York University Medical Center and the Weizmann Institute of Science in Israel generated a tremendous amount of excitement when they demonstrated the antiretroviral activity of hypericin and pseudohypericin.22 This preliminary study examined the effect of these compounds on two animal retroviruses, Friend leukemia virus and radiation leukemia virus, both in vitro and in vivo (in mice). The researchers found the effective dose of hypericin in mice to be 1.5 to 2 mg/mL. The researchers concluded the following:
Hypericin and pseudohypericin display an extremely effective antiviral activity when administered to mice after retroviral infection. The antiviral activity is remarkable both in its mechanism of action and in the potency of one administration of a relatively small dose of the compounds. Availability and the relatively convenient and inexpensive procedure for the extraction and purification of hypericin and pseudohypericin further enhance the potential of these compounds.
Later, two possible mechanisms were described to explain the antiviral activity of both hypericin and pseudohypericin.23 First was inhibition of assembly or processing of intact virions from infected cells—released virions contain no detectable activity of reverse transcriptase. Second, these compounds also directly inactivate mature and properly assembled retroviruses.
The antiviral activity of hypericin against human immunodeficiency virus (HIV) appears to require interaction with light to activate the hypericin.24,25 Another requirement is sufficient concentrations, as entry of hypericin into infected cells depends on the concentration of hypericin in the blood. At sufficient concentrations, hypericin incubated with HIV-infected whole blood decreases culturable HIV, indicating significant antiviral activity.26
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree