27
Hyaluronan-Based Autologous Chondrocyte Implantation
The marked increase in sports participation and increased emphasis on physical activity in all age groups has increased both the incidence of articular cartilage lesions and the recovery expectations of the patients. However, articular cartilage lesions are difficult to treat due to the distinctive structure and function of hyaline cartilage and to the difficulty in determining which lesion will be symptomatic and will evolve in degenerative joint changes. Curl et al1 found a 63% incidence of chondral lesion in a survey of 31,516 knee arthroscopies. Grade IV lesions were noted in 20% of patients and only 35% had no accompanying meniscal or ligamentous lesions. It is therefore difficult to determine which tissue injury is responsible for which symptoms and to what extent. Recently, Steadman et al2 reported highly satisfactory results at 11 years’ follow-up with the microfracture technique; however, patients may have to adjust their activity level to that of their knee function, and the authors stress the importance of a meticulous postoperative program that include the use of continuous passive motion (CPM) and 8 weeks of restricted weight bearing. Microfracture technique is simple and can be used in small lesions or in wide degenerative lesions; however, the repair tissue response can be unpredictable and variable, and it is unclear which stress is optimal for cartilage regeneration. Nehrer et al3 frequently found fibrous soft, spongiform tissue combined with central degeneration in the defect. Moreover, the clinical failure has been observed at a mean time of 21 months after treatment.
Autologous chondrocyte implantation (ACI) has been developed as a reconstructive technique with the aim of replacing the cartilage defect with a new developed mature cartilage tissue, trying to restore a complete normal joint. The clinical use of autologous chondrocyte implantation was pioneered in Sweden in 1987 to treat patients with chronic symptoms of cartilage lesions.4 The first clinical report in 1994 demonstrated highly satisfactory results with biopsy samples showing hyaline-like cartilage. Recently, Peterson et al5 showed durability of the early results obtained with ACI technique. In fact, after 2 years, 50 of 61 patients had good to excellent results. At 5 to 11 years’ follow-up, 51 of 61 patients had satisfactory results. Biomechanical evaluation of the grafted area by means of an indentation probe demonstrated stiffness measurements 90% that of normal cartilage. The outcome of these studies has demonstrated that 84 to 91% of the patients were able to achieve good to excellent results and return to active lifestyles. Therefore, we agree with Sgaglione et al6 that ACI is a safe, effective, and reproducible treatment that should be considered a viable option for young patients with cartilage lesions greater than 2 cm2 who want to resume an active lifestyle and restore so-called normal cartilage.
On the other hand, a recent study of Engebretsen7 comparing prospectively microfracture versus ACI has shown that Lysholm and Visual Analog Scale (VAS) pain scores improved in both groups at 2 years, and the Tegner score improved only in the microfracture group. Microfracture patients had fewer failures and reoperations than ACI patients. However, ACI patients had a better histologic quality of the repair tissue than did microfracture patients. This study has demonstrated that the ACI technique can achieve the restoration of cartilage tissue in the defect in ~85% of the cases, but there are still many biologic and technical factors that influence the final clinical outcome and lead to comparable clinical results, especially at short-term follow-up with a simpler technique, as reported by Engebretsen.7
Our efforts have been utilized in the attempt to improve the efficacy and reduce the morbidity of ACI technique, which still remains for us one of the main concerns of this technique. In the original ACI technique, the liquid cell suspension is difficult to handle during surgery, as it needs to be covered by a periosteal flap, and the surgical technique is tedious, time-consuming, and initially technically difficult. Moreover, there is a need for an arthrotomy for joint exposure. This factor increases morbidity especially for young athletes, increasing the risk of joint stiffness and arthrofibrosis frequently observed with this procedure. Micheli and coauthors8 in 2001, and other authors9 more recently, have shown a reoperation rate of up to 42% due to joint stiffness or hypertrophic changes of the implanted graft owing to the intrinsic growth capacity of the cambium layer of the periosteal flap9 with impingement syndrome as clinical findings. Another concern is whether the chondrocytes will be homogeneously distributed in the three-dimensional spaces of the defect10 when used in liquid cell suspension.
To avoid these technical problems, we have used a new tissue-engineering technology to create a cartilage-like tissue in a three-dimensional culture system with the possibility to reduce morbidity and improve cell culture biology. HYAFF® (Fidia Farmaceutici s.p.a., Abano Terme, Italy) is the class of hyaluronan derivatives obtained by esterifying the glucuronic acid group with different types of alcohols.11 HYAFF-11®—based scaffolds can be used in skeletal tissue engineering both as a tissue-guiding device and as a delivery vehicle.13 HYAFF-11 nonwoven matrix has been extensively characterized in a series of in vitro and in vivo studies in which it has been shown to effectively support growth of chondrocytes and to favor the expression of typical chondrocyte markers.14 This three-dimensional scaffold allows the maintenance of different phenotypes during culture and after implant. The quality of this scaffold in the laboratory experimental tests has facilitated verifying this system in an experimental animal trial. Grigolo et al13 in a rabbit model obtained statistically significant differences in the quality of the regenerated tissue found between the grafts performed with biomaterial carrying chondrocyte cells compared with the biomaterial alone or controls, thus demonstrating the efficacy of HYAFF-11-based scaffold for autologous chondrocytes implantation.
With this scientific and promising background, we have started to use ACI with HYAFF in symptomatic cartilage lesions. Due to the easy handling capacity of this scaffold, the implant can be performed by the mini-open or arthroscopic technique15 depending on the location of the defect.
Thanks to hydrophilic features of the scaffold, if the patch is correctly positioned inside the prepared defect, tensioactive pressure permits a natural fixation of the patch without the need of fibrin glue, or periosteal flap coverage. The avoidance of a periosteal flap enabled us to implant the chondrocyte-suspended scaffolds arthroscopically, simplifying and reducing the morbidity of this two-stage procedure.
 Surgical Technique
Surgical Technique
The arthroscopic surgical technique for ACI involves two separate procedures. The arthroscopic biopsy of healthy cartilage for cell culture remains mandatory to evaluate the site of the lesion and cartilage quality. At this time associated procedures such as anterior cruciate ligament (ACL) reconstruction or meniscal surgeries are usually performed.
In the second arthroscopic procedure, the lesion is visualized and, using a motorized shaver, debridement and lavage of the chondral lesion is performed. All fibrous tissue is removed from the surface of the lesion. Mapping and sizing of the defect is then executed using a delivery device of variable diameters (6.5–8.5 mm) with a sharp edge to achieve the complete coverage of the defect (Fig. 27–1). A flipped cannula especially designed is then inserted in the anteromedial portal. The flip allows removal of the fat pad from the field of view of the camera, especially when you have to put the knee in a high degree of flexion. A specifically designed cannulated low profile drill (6.5–8.5 mm) is positioned according to the mapping previously performed (Fig. 27–2). The drill is maintained in the selected position by a Kirschner guide wire (0.9-mm diameter) fixed in the bone. This drill, which presents a safety stop at a 2-mm distance, has been developed specifically to avoid damage to the subchondral bone plate, which has to remain intact during debridement of the lesion. Only the Kirschner wire passes through the subchondral plate, but the amount of stem cells coming from this small hole is not significant enough to require modifying the action of cultured chondrocyte. The low-speed drilling of the cartilage surface enables the surgeon to create the predetermined circular area with regular margins for the graft (Fig. 27–3). This step must be executed carefully to achieve stable and precise lesion contours.
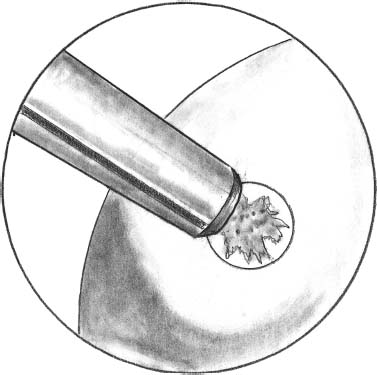
FIGURE 27–1 The sizing and mapping of the lesion is performed with the sharp edge of delivery system
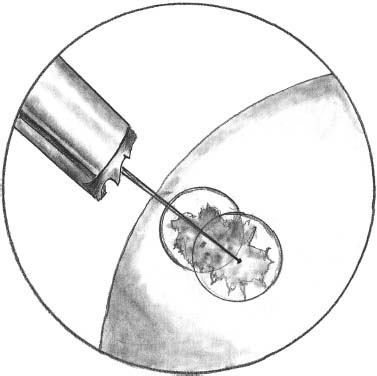
FIGURE 27–2 Preparation of the area is performed, with a low profile and slow speed drill, avoiding penetration of the subchondral bone.
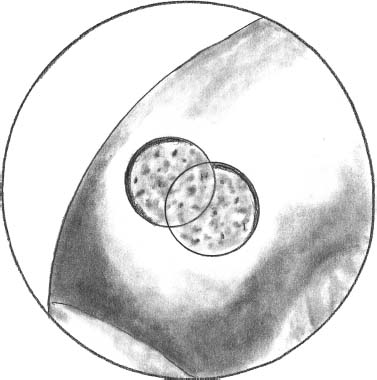
FIGURE 27–3 Prepared surface of the chondral lesion after drilling.
The procedure is repeated to prepare the entire defect surface according to the previous sizing. From the same portal, it is usually possible to correctly prepare a wide area, changing the knee flexion and orientation of the cannula thanks also to the previously mentioned flipped cannula.
After drilling, the joint is lavaged with a motorized shaver. The inflow then is closed and suction from the cannula inserted in the anteromedial portal is applied to create a dry joint surface.
The delivery system with a sharp edge is put in contact with the hyaluronic acid patch containing the autologous chondrocyte culture (Fig. 27–4). The stamp obtained remains automatically in the sheath of the delivery system, which is then transported through a cannula and positioned in the prepared area (Fig. 27–5). The delivery tamp is pushed to advance the stamp into the defect. The procedure is repeated until the entire defect is entirely filled (Fig. 27–6). It is important to cover the prepared area as much as possible without overhanging the margin of the defect with the implanted stamps. In this manner, the stamps do not move from the defect. This has been tested after repeated cycles of joint motion (with and without tourniquet) performed in open cases previously performed utilizing the same device.
Under arthroscopic visualization, the stability of implanted stamps is evaluated with a blunt probe. The tourniquet is released and the graft swelling is observed and the stability reevaluated. If the swelling of the patch increases its size in such way that the graft overhangs the margins of the defect, a smaller patch should be placed to prevent overhanging. For example, place a 6.5-mm diameter patch in a 8.5-mm diameter area. With the scope still in the joint, the knee is cycled several times, and the possibility of graft migration from the prepared defect is checked. Mobilization of the implanted patch has never been observed in our series. The arthroscopic implant has been developed for medial or lateral condyle lesions. With improving expertise and a long learning curve, we are now able to address almost any lesion, except for those in the patella.
FIGURE 27–4 The delivery system with a sharp edge is put in contact with the hyaluronic acid patch containing the autologous chondrocyte culture.
Rehabilitation Protocol
Patients are discharged on postoperative day 1 after the arthroscopic procedure. In the first 2 weeks, continuous self-assisted passive motion is started from 0 to 90 degrees from the second postoperative day, promoting joint nutrition and preventing adhesions. Stretching exercise and quadriceps contractions are allowed if tolerated. Toe-touch weight bearing is permitted, whereas full weight bearing is avoided for the first 4 weeks. From the 4th to 5th week, weight bearing is increased, beginning in the swimming pool, to recover the normal gait phases; muscle strengthening exercise is allowed from the 7th week. Increased strength and functional exercise is then gradually allowed. Return to contact sports should not be attempted before 8 to 12 months.
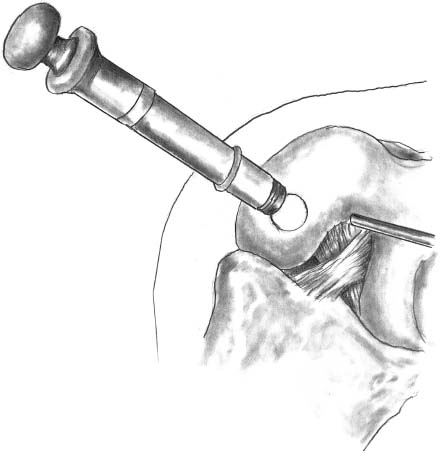
FIGURE 27–5 The tamp is pushed in the delivery system to precisely plug the stamp in the defect.
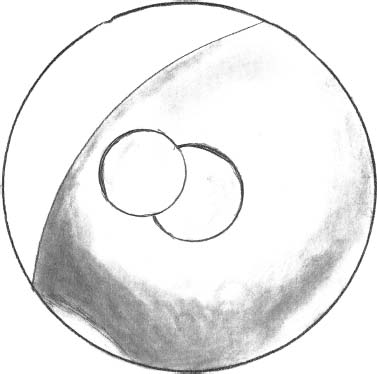
FIGURE 27–6 Complete coverage of the defect by two implanted patches after irrigation removal from the joint.
 Patient Selection and Prospective Follow-Up Evaluation
Patient Selection and Prospective Follow-Up Evaluation
The autologous chondrocyte transplantation on HYAFF scaffold has been used in Europe since 1999. At the moment, more than 3000 implants have been performed by the mini-open or arthroscopic techniques. At our institution, 139 patients have had this procedure since September 1999. Of these cases, 88 patients were treated arthroscopically (arthroscopic technique has been used since November 2000). The approval of the ethics committee of the Rizzoli Orthopaedic Institute was obtained for the clinical experimentation, and the informed consent of all the patients was obtained. All the patients were prospectively evaluated clinically according to the International Repair Cartilage Society score preoperatively and at 12, 24, and 36 months postoperatively. We were able to obtain CT or MRI scans for all patients at 12, 24, and 36 months of follow-up.
Among the patients who were treated by arthroscopic procedure, we have selected a group of 24 young athletes. Of these patients, 13 were soccer players, three skiers, three basketball players, one volleyball player, one rugby player, one body builder, one tennis player, and one biker. Of these athletes, eight practiced sports at the professional level. Twenty-four patients (23 male, one female) were analyzed at a minimum of 1-year follow-up. Twenty-two patients had isolated chondral lesions: 13 medial condyle, eight lateral condyle, and one trochlear lesion. Two patients had multiple knee lesions: one lateral compartment kissing lesion and one medial condyle and trochlea defect. All the defects were grade III to IV Outerbridge and the mean size was 2.9 cm2 (2–4.5 cm2). Mean age of the patients at time of surgery was 25 years (range 16–37 years). The etiology was traumatic in 18 cases, osteochondritis dissecans (OCD) in one case, and degenerative (microtraumatic) in five cases. Of the 18 traumatic lesions, seven were treated acutely (within 3 months after traumatic event) and 11 chronically. In 17 patients, associated procedures were performed during the cartilage harvesting: 15 ACL and one posterior cruciate ligament (PCL) reconstruction, nine medial and three lateral meniscectomies, two medial and one lateral meniscal repairs, and one autologous bone grafting. Previous surgery in 10 of the patients included three meniscectomies; two ACL reconstructions; one patellar tendon repair; five cartilage reparative operations, such as shaving and debridement of chondral lesion and one mosaicplasty.
Patients were asked for a subjective evaluation of the knee symptoms and physical function using the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form. According to this questionnaire, a higher score represents higher levels of function and lower levels of symptoms. Therefore, a score of 100 is interpreted to mean no limitations on activities of daily living or sports with the absence of symptoms. Patients were also asked to evaluate their quality of life using the EuroQol EQ-5D questionnaire. This is a recognized assessment of health—related quality of life based on self-care, mobility, usual activities, and pain/discomfort and anxiety/depression dimensions. It includes a 0 to 100 Visual Analogue Scale (EQ-VAS) for a self-rating of the global health state, in which the 100 value represents the best imaginable health state. A knee functional test was performed by the surgeon according to the IKDC Knee Examination Form. The lowest ratings in effusion, passive motion deficit, and ligament examination were used to determine the final functional grade of the knee (normal, nearly normal abnormal, or severely abnormal).
No complications related to the implant and no serious adverse events were observed during the treatment and follow up period. The International Cartilage Repair Society (ICRS) objective evaluation was normal or nearly normal in 100% of patients displaying knee conditions within the two best categories (Fig. 27–7).
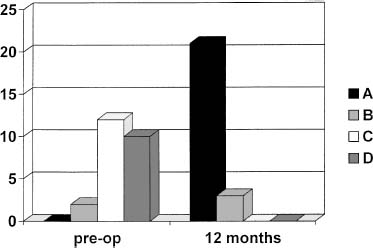
FIGURE 27–7 Clinical results in 24 patients at 12-month follow-up according to the ICRS objective evaluation.
The mean IKDC subjective score increased from 42.0 [standard deviation (SD) = 15.6] preoperatively to 80.8 (SD = 12.5) at 12-month follow-up. Subjective improvement in knee function and symptoms was seen in 95.8% of the patients. Only one patient (4.2%) experienced an unchanged knee condition. An improvement in quality of life, as assessed by the EQ-VAS, was noted in 87.5% of patients.
Twelve patients were analyzed at 12 and 24 months. The mean IKDC subjective score of these patients was 39.9 (SD = 19.3) preoperatively and 78.1 (SD = 15.0) at 12 months of follow-up and 84.5 (SD = 10.7) at 24 months (Fig. 27–8). None of these 12 patients worsened from 12 to 24 months’ follow-up.
Resumption of sports participation at the same or a slightly lower level was obtained in 14 (58.3%) of 24 patients at 12 months’ follow-up. Eight patients returned to sports activity at a lower level. Only two patients were not able to return to sports activity at 12 months. However, one of these two patients achieved nearly complete resumption of sports activity after 24 months. At 24 months, the resumption of sports activity was complete or nearly complete in 10 (83.3%) of the 12 patients analyzed (Fig. 27–9).
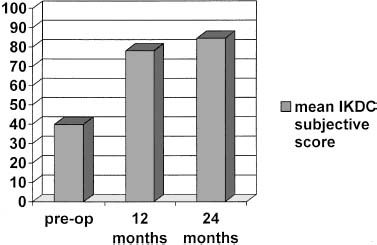
FIGURE 27–8 Clinical results in 12 patients at 12- and 24-month follow-up according to the IKDC subjective evaluation.
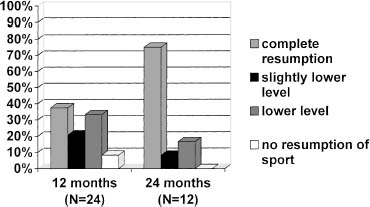
FIGURE 27–9 Resumption of sports activities of 24 patients at 12-month follow-up and of 12 patients at 24-month follow-up.

FIGURE 27–10 Histologic evaluation of regenerated tissue at 12-month follow-up. Excellent integration of the newly formed tissue with the subchondral bone. The tidemark is developing. (Courtesy of Prof. G. Abatangelo, University of Padova, Italy.)
A second-look arthroscopy was performed in five patients at a 12-month follow-up. Visual inspection and probing for the consistency of the implanted cartilage revealed complete healing of the defect and the excellent quality of regenerated cartilage macroscopically. The biopsy of implanted cartilage was performed in two cases, and the histologic evaluation by an independent examiner showed a hyaline-like tissue with good integration within the host tissue (Fig. 27–10). The results of our series are in agreement with those of the original ACI technique.4 The arthroscopic implant, however, has reduced the morbidity for the patient, the recovery time, and the rehabilitation protocol, allowing easier recovery for patients. This technique achieves clinical and histologic results comparable to those of traditional ACI, but reduces the morbidity for patients and improves the reliability of the procedure. Certainly, this technique is only one step forward from the original ACI technique. Several improvements will probably appear quickly as more knowledge on cell culture and chondrocyte behavior will permit a more reliable surgical technique and clinical outcome.
REFERENCES
1.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy 1997;13:456–460
2.Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy 2003;19:477–484
3.Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop 1999;365:149–162
4.Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop 2000;374:212–234
5.Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med 2002;30:2–12
6.Sgaglione NA, Miniaci A, Gillogly SD, Carter TR. Update on advanced surgical techniques in the treatment of traumatic focal articular cartilage lesions in the knee. Arthroscopy 2002;18(suppl 1):9–32
7.Engebretsen L. Comparison ACT—microfractures. Proceedings of the International Symposium on Matrix-Assisted Chondrocyte Transplantation—a 2-year-update with hyalograft C. Bad Gastein, Austria, January 23–25, 2004
8.Micheli LJ, Browne JE, Erggelet C, et al. Autologous chondrocyte implantation of the knee: multicenter experience and minimum 3-year follow-up. Clin J Sport Med 2001;11:223–228
9.Anderson AF, Fu FH, Bert RM, et al. A controlled study of autologous chondrocyte implantation versus microfracture for articular cartilage lesions of the femur. 70th American Academy of Orthopaedic Surgeons (AAOS) annual meeting proceedings, New Orleans, February 5–9, 2003
10. Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br 2002;84:571–578
11. Campoccia D, Doherty P, Radice M, Brun P, Abatangelo G, Williams DF. Semi synthetic resorbable materials from hyaluronan esterification. Biomaterials 1998;19:2101–2127
12. New frontiers in medical sciences: redefining hyaluronan. In: Abatangelo G, Weigel PH, eds. Proceedings of the Symposium Held in Padua, Italy—17–19 June 1999. New York: Elsevier, 2000
13. Grigolo B, Roseti L, Fiorini M, et al. Transplantation of chondrocytes seeded on a hyaluronan derivative (HYAFF®11) into cartilage defects in rabbits. Biomaterials 2001;22:2417–2424
14. Brun P, Abatangelo G, Radice M, et al. Chondrocyte aggregation and reorganization into three-dimensional scaffolds. J Biomed Mater Res 1999;46:337–346
15. Marcacci M, Zaffagnini S, Kon E, Visani A, Iacono F, Loreti I. Arthroscopic autologous condrocyte transplantation: technical note. Knee Surg Sports Traumatol Arthrosc 2002;10:154–159
< div class='tao-gold-member'>



