Heterotopic Bone Formation
Lawrence M. Specht
William L. Healy
Introduction
Heterotopic ossification (HO), which was first described by Riedel in 1883 (1), is formation of mature lamellar bone at an ectopic site in the body (2,3) because of inappropriate differentiation of fibroblasts into bone-forming cells. HO can occur in any soft tissue, other than periosteum, but most commonly it is found in muscle following surgical trauma. In contrast, myositis ossificans (MO) occurs following trauma, and only in muscle. HO can develop following local tissue trauma, and it can be associated with peripheral nerve injuries (sciatic), spinal cord injury, and infection. HO can be responsible for pain and limitation of motion.
Case
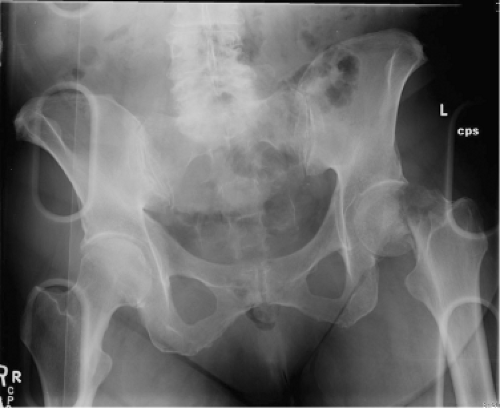
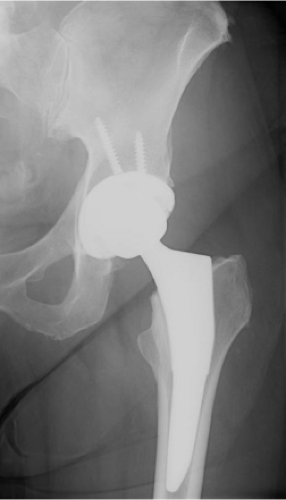
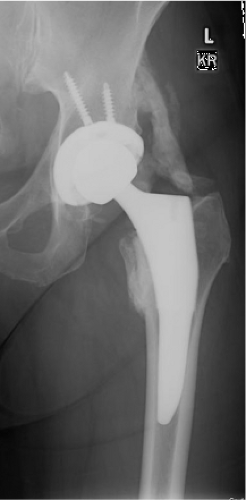
A 67-year-old community ambulatory female sustained a left displaced femoral neck fracture (Fig. 89.1). She also had an ipsilateral nondisplaced olecranon fracture managed nonoperatively. After medical optimization, she was taken to the operating room for treatment with a cementless total hip arthroplasty (THA) using an anterior approach (Fig. 89.2). The periarticular tissues were injected with local anesthetic, duramorph and toradol. A drain was placed in the wound. She was treated with adjusted dose Coumadin for VTE prophylaxis. Her hospitalization was uncomplicated. The wound was dry, and she remained within the goal INR range.
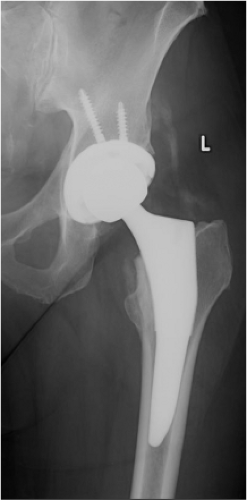
Two weeks after discharge, she was progressing well clinically with occasional pain in her thigh. An x-ray was obtained (Fig. 89.3) that showed satisfactory implant position with no subsidence and density consistent with calcification. At this time, warfarin was discontinued, and aspirin was started per VTE prophylaxis protocol.
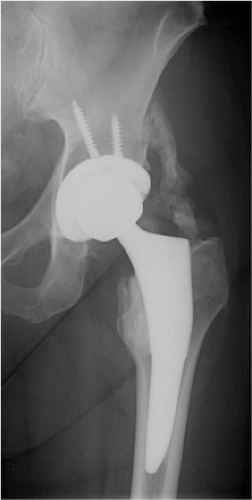
Six weeks following operation (Fig. 89.4), she reported an increase in her pain about the hip and thigh to 6/10 on a VAS, physical examination noted decreased ROM with ADLs, but able to flex to 80 to 90 degrees. Early calcification was noted on x-ray shown in Figure 89.2. Her alkaline phosphatase was 169 (NL 30 to 115).
At 3 months she continued to have pain and stiffness. Her ROM was painful with flexion >60 degrees, 5 IR, 30 ER, 20 ABD, and 10 ADD. X-ray showed HO extending nearly from ilium to tip of the trochanter. Patients CRP was normal and alkaline phosphatase remained elevated (Fig. 89.5). Patient counseling and education was continued.
Clinical Problems/Presentation
The first sign of developing HO is usually pain and inflammation at the site of operation in the early postoperative period. This inflammation persists, unlike the usual convalescent period. In the absence of pharmacologic measures to delay ossification, HO becomes visible 3 to 4 weeks postoperatively. It begins as wispy calcifications on x-ray and may progress to bridging bone. Although NSAIDs and analgesics will help with the symptoms, they will not alter the ultimate course. As the ossification progresses, joint stiffness may develop. HO may form an impinging mass about the hip, which can lead to impingement and dislocation despite well-positioned implants and appropriate reconstruction. It is important in this early period to differentiate pain, warmth, and inflammation of HO from infection and other
sources of pain. These two complications are not mutually exclusive.
sources of pain. These two complications are not mutually exclusive.
Maturation of HO occurs within 6 months to 2 years. This process can be monitored on serial radiographs or through bone scintigraphy to assess metabolic activity. Increased soft tissue uptake is seen as soon as 3 to 4 weeks postoperatively. The scintigraphy is looking specifically at the soft tissues around the hip as the bony changes may continue for 1 to 2 years in a well-functioning arthroplasty. The HO image becomes “cold” when remodeling is complete. The HO image becomes “cold” when remodeling is complete and normalization of local metabolism returns along with measured bone turnover metabolites such as alkaline phosphatase and urinary deoxypyridinoline (D-PYD), then the remaining symptoms are related to the stiffness and/or impingement. Excision of this periarticular tissue may improve ROM and eliminate the source of impingements. Alternatively, excision of the HO and periarticular tissues may lead to loss of soft tissue constraints, leading to the (apparent) need for modular lengthening as well as neurovascular and clinical risk.
Algorithm to Diagnosis
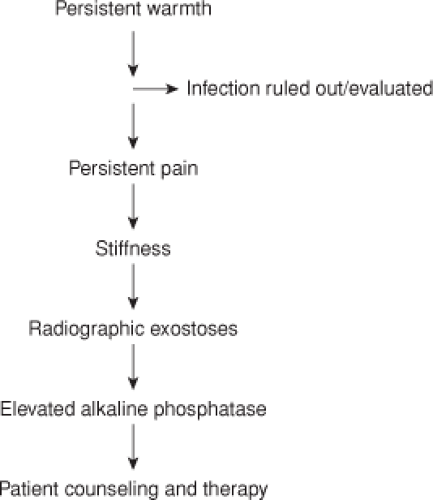
The key to proper diagnosis is a high clinical suspicion based on preoperative risk factors listed above (Table 89.1) (Fig. 89.6). Although many surgeons obtain their first postdischarge x-rays at 6 weeks, a patient who is not progressing as expected at the 2-week wound check warrants evaluation. This may be as simple as a more frequent follow-up visit or may include screening radiographs, blood work including alkaline phosphatase, and infection markers. A carefully documented examination at this early visit allows one to note clinical improvement or failure to progress. Evidence of stiffness and pain or warmth beyond normal warrants further attention and patient discussion.
Classification System
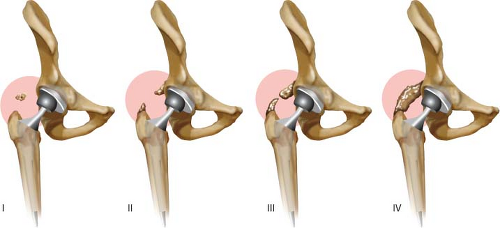
Among several classifications of HO (4,5,6,7) the most commonly used is the Brooker classification, this is based on the appearance of ossification on AP hip radiographs Table 89.2. Grade 0 has no ectopic bone formation seen on AP x-ray. Grade 1 consists of isolated islands of bone in the soft tissues less than 1 cm (Fig. 89.7). In grade 2, exostoses extend from the pelvis or femur (Fig. 89.7) and more than 1 cm separates these. In grade 3, these exostoses are separated by less than 1 cm on the AP x-ray (Fig. 89.7). Grade 4 represents radiographic ankylosis of the hip; though not necessarily complete clinical ankyloses (Fig. 89.7). Brooker grades 1, 2, and 3 are associated with few clinical symptoms. Grade 4 is typically symptomatic.
Table 89.1 Risk Factors for Developing HO | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 89.2 Brooker Classification for Describing HO on AP Film | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||
In an effort to improve reliability and reproducibility DellaValle et al. proposed a modification to the Brooker classification which decreased from four to three categories. Grade A includes complete absence of HO or isolated islands less than 1 cm in length. Grade B is one or more islands at least 1 cm in length and exostoses from femur and pelvis with >1 cm between opposing surfaces. Grade C has less than 1 cm between opposing surfaces or apparent ankylosis.
Both of these systems rely on AP pelvis x-rays and have not included a volumetric assessment or a consideration of the clinical implications on range of motion or pain (4,8). CT and three dimensional reconstruction can be used to develop a volumetric calculation to objectify the performance of prophylactic regimens (9). This is most helpful for research or for preoperative planning of excision.
Hamblen et al. (10) developed a system that graded HO by categorizing the size, location, and effect on hip range of motion. This system has not been widely adopted. Maloney (11) modified the Brooker classification to reflect functional limitations that result from HO. They subdivided grade 3 and 4 into A and B. In A, there was no difficulty with sitting, ascending stairs, or putting on shoes and socks. In B, the HO caused sufficient limitations to cause difficulties with these activities/ADLs. This may be the most useful way to approach your patient with HO.
Pathophysiology
The development of HO can have traumatic, neurologic, and genetic causes. These may overlap. HO is initiated by an unknown trigger that causes primitive mesenchymal cells, which are present in muscle, fascia, periosteum, and bone marrow, to differentiate into osteoprogenitor cells. These cells may be present locally, or be delivered to the soft tissue by trauma of surgery: acetabular reaming and femoral broaching. These cells are induced to form osteoblastic tissue. The osteoid matrix is laid down and eventually calcified. The transformation of these primitive mesenchymal cells into osteoblastic tissues may occur within 16 hours after the index surgical procedure. Maximal stimulus for HO formation is reached by 32 hours after surgery. Based on the pathophysiology of HO, optimal HO prophylaxis should be instituted less than 4 hours preoperatively or within 24 to 48 hours postoperatively (12,13,14). HO prophylaxis can still be effective within the first 4 days after surgery (14).
Growth factors such as bone morphogenic proteins (BMP) stimulate HO transformation. BMP-4 has been shown to be over expressed in a genetic form of HO and is blocked by the BMP antagonist Noggin. Similarly, Prostaglandin E2 is likely involved. This explains the prophylactic effectiveness of indomethacin and naproxen, two prostaglandin inhibitors. The growth factors are likely released into local tissues after tissue trauma or brought in from local reamings. Angiogenesis plays a role in the development of this ectopic bone through angiogenesis factors (15). Endothelial cytokine have effects on the differentiation of osteoprogenitor cells and differentiation of pericytes into osteoblastic and chondroblastic cell lines (16).
HO in soft tissues undergoes extensive remodeling as the tissues mature. Osteoclastic and osteoblastic activity occurs concurrently, but the osteoblastic activity prevails. HO increases in mass during the first 1 to 2 years postoperatively before stabilization occurs. During this phase, many of the symptoms of warmth, inflammation, and accompanying pain are related to this metabolic activity. Once this maturation occurs, the remaining symptoms are related to the mass effect of the resulting HO.
Reported rates of HO following THA range from 2% to 90% depending upon the criteria of diagnosis and risk factors of the population under study (17). Although HO of the hip is commonly associated with surgery, it can occur without local trauma such as after spinal or head injury (18




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree