Hepatic Steatosis
William J. Cochran
Hepatic steatosis is a common entity that frequently goes unrecognized in the pediatric population. The term hepatic steatosis is used to describe excessive fat in the liver. Normally, fat accounts for less than 5% of the weight of the liver. Hepatic steatosis is not a primary disease process and can occur in many situations. Steatohepatitis is the presence of excessive hepatic fat in conjunction with inflammation. Ludwig in 1980 first used the expression nonalcoholic hepatic steatohepatitis (NASH) to
describe steatohepatitis not associated with excessive alcohol consumption. Subsequently this term has been dropped in favor of nonalcoholic fatty liver disease (NAFLD) to describe the spectrum of liver disease associated with excessive hepatic fat. NAFLD associated with obesity in adults has been recognized for almost 50 years. Moran was the first to report this entity in pediatric patients in 1983.
describe steatohepatitis not associated with excessive alcohol consumption. Subsequently this term has been dropped in favor of nonalcoholic fatty liver disease (NAFLD) to describe the spectrum of liver disease associated with excessive hepatic fat. NAFLD associated with obesity in adults has been recognized for almost 50 years. Moran was the first to report this entity in pediatric patients in 1983.
PATHOPHYSIOLOGY
Fat accumulates in the liver in a complex and, in certain situations, unknown manner. Although a complete discussion of hepatic lipid metabolism is beyond the scope of this chapter, several excellent reviews exist. Fat accumulation usually results from increased uptake, increased synthesis, decreased oxidation, or decreased secretion of fat by the liver.
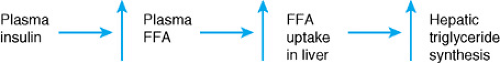
Uptake of free fatty acids by the liver is proportional to the amount of free fatty acids to which the liver is exposed (Fig. 372.1). Ingestion of carbohydrates, especially glucose and fructose, as well as increased uptake of free fatty acids promotes hepatic triglyceride synthesis (Fig. 372.2). If triglyceride release in the form of lipoproteins does not increase proportionally, fat accumulates in the liver. Several factors are associated with increased plasma free fatty acid levels: acute starvation, elevation of several hormones (e.g., adrenocorticotropic hormone, thyrotropin, thyroid hormone, growth hormone, insulin), obesity, and use of certain drugs (e.g., corticosteroids, epinephrine).
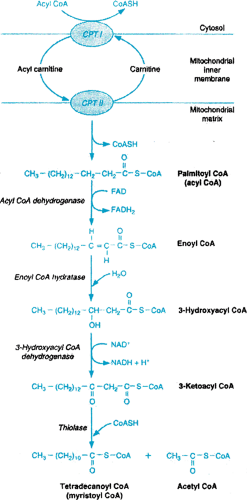
A reduction in the oxidation of fatty acids may also result in development of hepatic steatosis. This can occur secondary to ingestion of chemicals such as hypoglycin A or amanitotoxin as well as to defects in fatty acid metabolism such as long-chain fatty acyl dehydrogenase deficiency (Fig. 372.3). Obesity is now the most common entity associated with NAFLD; therefore, its pathogenesis deserves further elaboration. The pathogenesis of NAFLD is felt to result from two metabolic alterations—hyperinsulinemia (Fig. 372.1) and increased oxidative stress, which is thought to be a cause of the inflammation in NAFLD and may be caused by increased fatty acids, which are hepatotoxic in their own right and also adversely affect intracellular membranes.
Many obese children have hyperinsulinemia. Elevated insulin levels promote an increase in plasma free fatty acids, which, as noted above, leads to increased uptake of free fatty acids by the liver and subsequently increased hepatic triglyceride synthesis. One means by which the liver can reduce its fat content is by re-esterification of fatty acids and subsequent secretion of triglycerides from the hepatocytes into the blood. There is some evidence that obese individuals may have a decreased ability to accomplish this. Together, increased fatty acid uptake by the liver due to hyperinsulinemia and decreased ability to remove fat from the liver would result in increased hepatic fat. Support for the importance of oxidative stress in this process is derived from a study documenting improvement in children with NAFLD treated with the antioxidant vitamin E.
Disorders Associated with Hepatic Steatosis
The various disorders often associated with hepatic steatosis can be divided into several broad categories: nutritional, toxinor drug-induced, metabolic, endocrine, infectious, and idiopathic (Box 372.1).
Hepatic steatosis is associated with several nutritional disorders, the first of which—protein-calorie malnutrition—was noticed by Williams in 1933. Hepatic steatosis is uncommon in patients with marasmus (i.e., protein and calorie deprivation) but common in patients with kwashiorkor (i.e., protein deprivation), in whom fat can account for 50% of the weight of the liver. The exact mechanism of hepatitic steatosis is unknown but is probably multifactorial, including elevation in the level of free fatty acids, reduction in peroxisomes associated with decreased oxidation of fat, decrease in apolipoprotein synthesis, and decrease in lipoprotein secretion. These patients frequently develop hepatomegaly, and their liver enzyme levels may be mildly elevated. Fat deposition begins first in the periportal area and progresses out to the central vein. With appropriate nutritional therapy, the fat disappears, first from the central area and last from the periportal area. Other isolated nutritional deficiencies, such as pyridoxine or riboflavin deficiency, may be associated with hepatic steatosis, although this is uncommon.
BOX 372.1 Disorders Associated with Hepatic Steatosis
Nutritional
Undernutrition (kwashiorkor)
Obesity
Carnitine deficiency
Choline deficiency
Toxin or drug induced
Aflatoxin
Amanitotoxin
Hypoglycin A
Carbon tetrachloride
Dimethylformamide
Total parenteral nutrition
Vitamin A
Corticosteroids
Alcohol
Tetracycline
Valproic acid
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree