Hemolytic Anemias
Paul L. Martin
After release from the bone marrow, mature, nonnucleated erythrocytes survive for 100 to 120 days in the circulation. In the steady state, 1% of the senescent erythrocytes is destroyed daily and replaced by an equal number of new erythrocytes released from the bone marrow. The basic pathophysiology of the hemolytic anemias is a reduced erythrocyte life span, ranging from nearly normal to remarkably shortened. In compensation, the bone marrow increases its output of erythrocytes, a response mediated by increased production of erythropoietin. In adults with hereditary spherocytosis (HS), the bone marrow can increase output of erythrocytes sixfold to eightfold. With this maximal response, erythrocyte survival can be reduced to only 20 to 30 days without the onset of anemia (i.e., compensated hemolysis). The limits of erythrocyte production in some hemolytic states have not been determined, particularly in infants and children.
A hemolytic process can be measured directly by erythrocyte survival rates or indirectly by the presence of increased levels of the metabolic products of hemolysis. Alternatively, a hemolytic process may be inferred by documentation of the increase in erythrocyte production that usually accompanies hemolytic states.
In response to shortened survival of erythrocytes, the activity of bone marrow increases, and the reticulocyte count exceeds 2%. Sustained reticulocytosis is presumptive evidence of hemolysis. Hyperplasia of the erythropoietic marrow elements occurs, with reversal of the myeloid-to-erythroid ratio from the normal 3:1. In the severe, chronic hemolytic processes of
childhood, hypertrophy of the marrow may expand the medullary spaces and produce bone changes, particularly in the skull and hands.
childhood, hypertrophy of the marrow may expand the medullary spaces and produce bone changes, particularly in the skull and hands.
Elevations of unconjugated bilirubin often occur in children with hemolytic anemias. However, overt jaundice may be absent. Bilirubin levels in excess of 5 mg/dL are unusual if liver function is normal. Chronic hemolysis is associated with increased excretion of bilirubin pigments leading to pigmented gallstones that may develop in early childhood.
In any hemolytic state, hemoglobin is released into the plasma, where it combines irreversibly with serum haptoglobin. The large complex is rapidly cleared from the circulation. When haptoglobin use exceeds synthesis, serum levels are decreased (less than 20 mg/dL).
Besides these indirect indicators of hemolysis, isotopic techniques can measure erythrocyte survival directly. Sodium chromate is most often used as an erythrocyte tag. A shortened erythrocyte survival is likely when the chromium half-time is reduced to less than 20 days. Such survival studies are rarely needed in clinical practice.
The hemolytic disorders may be conveniently and fairly accurately classified according to whether the shortened erythrocyte survival is a result of an intrinsic abnormality of the erythrocyte or an extrinsic abnormality acting on a normal erythrocyte. Intrinsic hemolytic anemias generally result from inherited abnormalities of the erythrocyte membrane, intracellular enzymes, or hemoglobin. Extrinsic disorders usually are acquired and result from forces or agents that immunologically, chemically, or physically damage the erythrocyte. These two categories are not mutually exclusive, and some hemolytic disorders are caused by a combination of intrinsic and extrinsic mechanisms (Table 291.1).
INTRINSIC HEMOLYTIC ANEMIAS
Hereditary Spherocytosis
HS occurs predominantly in people of northern European ancestry, although it has been found in patients of many ethnic groups. The typical features are a familial hemolytic anemia of various degrees of severity, splenomegaly, and spheric erythrocytes found on the blood smear.
In approximately three-fourths of patients, pedigree analysis indicates an autosomal dominant transmission. Sporadic dominant mutations have been invoked, and autosomal recessive transmission is present in some cases. The gene for some patients with HS is located on chromosome 8.
TABLE 291.1. CAUSES AND TREATMENTS OF HEMOLYTIC ANEMIAS | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pathophysiology
A deficiency or abnormality of the erythrocyte membrane structural protein spectrin appears to affect most patients with HS. Other erythrocyte membrane structural proteins that have been demonstrated to play a role in this disease include ankyrin, band 3, and protein 4.2. They all have in common the relative deficiency of spectrin. This deficiency is associated with an accelerated loss of the erythrocyte membrane, which reduces the erythrocyte surface area. Because no concomitant loss of cellular volume occurs, the erythrocytes become spheric.
The spleen is intrinsically involved in the hemolytic process. The splenic circulation imposes a metabolic stress on spherocytic cells. The spherocyte is relatively rigid and passes with difficulty through the splenic cords and sinuses. This results in their sequestration and destruction. The hemolytic process regresses after splenectomy, although biochemical and morphologic abnormalities persist.
Clinical Manifestations
The disease may present in the neonatal period with anemia and hyperbilirubinemia that may require phototherapy or exchange transfusion. The anemia varies considerably in severity but tends to be similar within the same family. The patient usually has slight jaundice. Expansion of the marrow cavity occurs to a lesser extent than in thalassemia. The spleen is almost always palpably enlarged after 2 or 3 years of age. Pigmentary gallstones have occurred as early as 4 years of age. Aplastic crises associated with parvovirus infections are the most serious complications during childhood. Other complications have been reported and are less common, including gout, leg ulcers, and growth retardation.
Laboratory Findings
Indicators of hemolysis include reticulocytosis, anemia, and hyperbilirubinemia. The hemoglobin level ranges from 6 to 10 g/dL, and the reticulocyte count ranges from 5% to 20% (average, 10%). The mean corpuscular hemoglobin concentration usually is elevated (greater than 36%). The spherocytic erythrocytes are smaller than normal erythrocytes and lack the central pallor of the biconcave disc, but only relatively small proportions of the cells are spherocytic. Erythroid hyperplasia occurs in the marrow, but erythrocyte precursors are not spherocytic.
Abnormality of the erythrocyte can be demonstrated by osmotic fragility studies. This test measures the ability of red blood cells to swell and increase their volume when they are subjected to varying degrees of hypotonic environments.
Because of their decreased surface-to-volume ratio, spherocytes have a limited capability of doing so and will be lysed at a higher salt concentration than normal cells. In 10% to 20% of patients with HS, the osmotic abnormality can be demonstrated only if the blood is incubated at 37°C for 24 hours.
Because of their decreased surface-to-volume ratio, spherocytes have a limited capability of doing so and will be lysed at a higher salt concentration than normal cells. In 10% to 20% of patients with HS, the osmotic abnormality can be demonstrated only if the blood is incubated at 37°C for 24 hours.
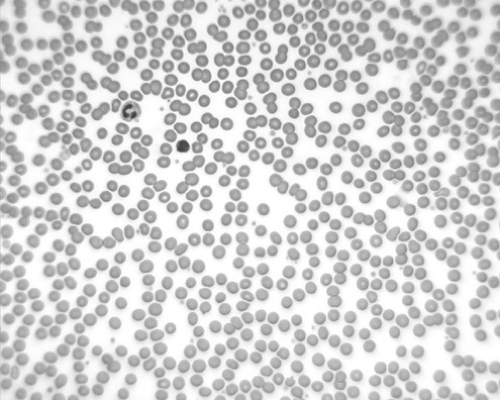
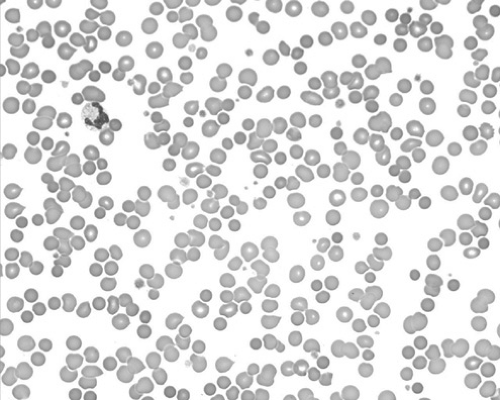
HS must be differentiated from other congenital hemolytic states. Family history, blood smear, and osmotic fragility studies offer the most diagnostic value. Acquired spherocytosis of the erythrocytes is seen in autoimmune hemolytic anemias (AIHAs), in which the spherocytosis is often more pronounced than in HS, and the direct Coombs test result is positive (Figs. 291.1 and 291.2). It may be difficult to differentiate HS from hemolytic disease caused by ABO incompatibility in the newborn infant. A period of observation may be necessary to clarify the diagnosis.
Therapy
Splenectomy almost invariably produces a clinical cure, although in a few instances of severe HS with recessive transmission, the operation is not curative. Splenectomy should be deferred if possible until the patient is at least 5 or 6 years of age. Immunization with conjugated pneumococcal and Haemophilus influenzae type b vaccine as recommended for all infants in the United States should be provided. In addition, polyvalent pneumococcal vaccine should be given at 2 years of age, with a boost at age 7 years. If anemia is severe enough to impair growth or normal activity, the operation can be considered earlier, after a period of observation. Splenectomy prevents further gallstone formation and eliminates the threat of aplastic crises.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree