Haemophilus Influenzae
Sheldon L. Kaplan
Haemophilus influenzae is a fastidious, gram-negative, pleomorphic coccobacillus that is responsible for serious systemic and local infections in children.
MICROBIOLOGY
H. influenzae is differentiated from other Haemophilus species by its requirement for factors X (i.e., heat-stable hematin) and V [i.e., heat-labile nicotinamide adenine dinucleotide (NAD)] for growth. Encapsulated strains of H. influenzae are classified by capsular polysaccharides types a through f. The polysaccharides are negatively charged, high-molecular-weight polymers that comprise repeating subunits of a disaccharide. In the prevaccine era, approximately 95% of invasive diseases were caused by the type b strain, for which the repeating subunit is polyribosyl ribitol phosphate (PRP). The partial deletion of IS1006-bexA, an insertion element, appears to be associated with increased capsule production and thus virulence for serotypes b and a. Unencapsulated strains (i.e., nontypable strains) primarily are etiologic agents in upper respiratory tract infections such as otitis media and sinusitis, but they also cause systemic disease, especially in the neonate or immunocompromised host.
H. influenzae organisms have several outer membrane proteins (OMP) that can be differentiated by sodium dodecylsulfate, polyacrylamide gel electrophoresis. Based on the OMP pattern, 21 subtypes of H. influenzae type b (Hib) can be described, although five subtypes account for more than 90% of the systemic isolates. OMP patterns have proved useful for epidemiologic investigations. Unlike type b strains, the OMP patterns of nontypable H. influenzae are highly variable. Hib strains also have been characterized by the electrophoretic mobility of 17 metabolic enzymes (i.e., multilocus enzyme electrophoresis). Most invasive disease in the United States is caused by clonal isolates of two related multilocus genotypes. H. influenzae isolates can be characterized into two primary phylogenetic divisions with serotypes c, e, and f capsules belonging to a single division and serotypes a and b occurring in divisions I and II.
H. influenzae contains a lipopolysaccharide that differs from lipopolysaccharides of Enterobacteriaceae in that it lacks
repeating O side chains and is classified as a lipooligosaccharide. The lipid A component and core oligosaccharide of H. influenzae lipopolysaccharide are similar in structure to enteric lipopolysaccharide. Lipopolysaccharide has a role in the adherence and colonization of the respiratory tract by H. influenzae organisms.
repeating O side chains and is classified as a lipooligosaccharide. The lipid A component and core oligosaccharide of H. influenzae lipopolysaccharide are similar in structure to enteric lipopolysaccharide. Lipopolysaccharide has a role in the adherence and colonization of the respiratory tract by H. influenzae organisms.
H. influenzae produce an IgA1 protease that can cleave specifically the hinge region of the heavy chain of human IgA1. Both typeable and non-typeable H. influenzae have at least 5 major adhesins (high molecular weight proteins (HMW) 1 and 2, Haemophilus adherence and penetration protein (Hap), Hia/Hsf and hemagglutinating pili. These adhesins are involved in attachment and colonization as well as invasion of the respiratory tract.
H. influenzae type b was the first organism for which the entire bacterial genome was sequenced enabling scientists to more completely study virulence factors and host-pathogen interactions.
EPIDEMIOLOGY
In the United States, approximately 20,000 cases of systemic Hib infection occurred yearly before the introduction of the Hib protein conjugate vaccines. Most cases were bacterial meningitis. The estimated annual age-specific attack rate of Hib infection was 100 cases per 100,000 children younger than 5 years of age. The highest age-specific attack rate occurred in children between the ages of 6 and 11 months; the next highest rate was among those between the ages of 12 and 17 months. Overall, approximately one in 200 children developed a systemic infection due to Hib by the time they reached 5 years of age.
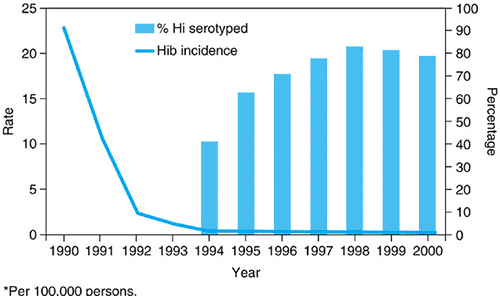
After the administration of the conjugate Hib vaccines to infants became routine, the incidence of invasive Hib infections in children fell dramatically. In Centers for Disease Control and Prevention (CDC) surveillance studies in the United States, conducted during 1998 to 2000, a total of 824 cases of invasive H. influenzae disease was reported among children younger than 5 years old; rates were 1.4 to 1.6 per 100,000 children. Of the isolates available for serotyping, only 22% were Hib in 2000, when the annual invasive disease rate for children younger than 5 years old was 0.3 per 100,000, a 99% decline in invasive Hib disease compared with the rate in 1990 (Fig. 161.1). The estimated incidence of invasive H. influenzae type b disease in US children in 2004 was 0.09 cases/100,000 for children <5 years old. As a result, nontypable strains and other non-b serotypes (especially type f) now cause a higher proportion of invasive H. influenzae infections. Serotypes a and f have been associated with meningitis and pneumonia in children younger than 5 years of age; 25% or more have an underlying illness.
Certain risk factors for systemic Hib infections have been identified. African American, Hispanic, and Native American children have higher rates of infection than do white, non-Hispanic children. The highest endemic incidence of disease in the prevaccine era occurred among native Alaskan Eskimos. Children younger than 4 years of age, who are household contacts of a patient with Hib disease, have a much higher risk for acquiring this disease than does the general population. Children with underlying immune deficiencies and anatomic or functional asplenia (e.g., hemoglobinopathies) are more likely to develop systemic H. influenzae infections. Other risk factors for the development of invasive Hib infections are attendance in day-care facilities, crowded households, frequent infections, and socioeconomic status. Additional risk factors for developing local infections caused by nontypable H. influenzae include viral respiratory infections, allergies, exposure to smoke, and anatomic abnormalities like cleft palate.
Breast-feeding for infants between 2 and 5 months of age appears to be a relatively protective factor.
PATHOGENESIS
Unencapsulated H. influenzae are common inhabitants of the upper respiratory tract under normal conditions in children and adults. Hib could be isolated from as many as 5% to 7% of young children at any time in the prevaccine era. Higher colonization rates occurred among children in day-care centers; much lower rates are observed in children after immunization with the H. influenzae protein conjugate vaccines.
Invasive disease caused by Hib frequently follows a viral upper respiratory infection, which may disrupt mucosal barriers and interrupt the normal activity of respiratory cilia. In infant rats, prior intranasal inoculation with influenza virus promotes bacteremia after the intranasal administration of Hib. In human nasopharyngeal organ cultures infected with Hib, organisms can be identified within the epithelium in an intercellular location by 24 hours. This invasion is preceded by disruption of the tight junctions between nonciliated cells. Presumably, after it passes the mucosal barrier, the organism can invade the bloodstream directly. In a susceptible host, bacteria multiply readily, and after a critical bacterial density is reached, dissemination occurs.
For local infections caused by unencapsulated strains, a preceding viral upper respiratory infection frequently disrupts the normal physiologic clearance mechanisms and permits
invasion of the sinuses or middle ear by normal respiratory flora (e.g., S. pneumoniae, nontypable H. influenzae, and Moraxella catarrhalis). However, certain nontypable strains may possess one or more virulence factors that may mediate bloodstream invasion and the erosion of host defenses and help explain why invasive infections caused by nontypable H. influenzae may occur. Nontypable strains can colonize the female genital tract and thus be associated with neonatal infection. Often, acute chorioamnionitis and villitis are present in the placenta of mothers whose infants have early-onset sepsis.
invasion of the sinuses or middle ear by normal respiratory flora (e.g., S. pneumoniae, nontypable H. influenzae, and Moraxella catarrhalis). However, certain nontypable strains may possess one or more virulence factors that may mediate bloodstream invasion and the erosion of host defenses and help explain why invasive infections caused by nontypable H. influenzae may occur. Nontypable strains can colonize the female genital tract and thus be associated with neonatal infection. Often, acute chorioamnionitis and villitis are present in the placenta of mothers whose infants have early-onset sepsis.
HOST IMMUNITY
Antibody to the capsular polysaccharide is the major component of the host defense against Hib. The inverse relation between PRP antibody concentrations and susceptibility to systemic infection is well known. Children who develop serum anti-PRP antibody concentrations of at least 1 μg/mL 3 weeks after immunization with PRP are protected against invasive disease. Antibody to PRP is an important opsonin, is bactericidal in combination with complement, and promotes neutrophil chemotaxis. Functional differences exist for IgG and IgM and for the IgG subclasses (e.g., IgG1, IgG2), antibodies directed against PRP, but the relative contributions of the immunoglobulin classes to host defenses are unclear. Mucosal anti-PRP antibody (IgA) develops after natural diseases and parenteral immunization with PRP, but the clinical significance of IgA anti-PRP is unknown.
Antibodies to other noncapsular polysaccharide antigens, such as OMPs and lipooligosaccharides, may play some role in immunity against H. influenzae. Hib lipooligosaccharide is an important inducer of the inflammatory response, related to the production of cytokines, such as tumor necrosis factor (TNF) or interleukin-1 (IL-1). The macrophages of the reticuloendothelial system are key components for intravascular clearance of Hib.
Several surface antigens (ex. HMW1/HMW2) have been considered as vaccine candidates in studies of immunity to nontypable H. influenzae.
CLINICAL MANIFESTATION AND COMPLICATIONS
Infections Caused by Haemophilus influenzae Type B
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree