CHAPTER 13
Guidance Techniques for Botulinum Toxin Injections: A Comparison
Katharine E. Alter
Muscle overactivity including spasticity, dystonia, other forms of hypertonia, and movement disorders are common impairments affecting patients with neurologic conditions (1). Treatment of problematic muscle overactivity (PMOA) includes physical therapy, splinting/casting, enteral medications, injectable/chemodenervation therapy, and surgical options, including intrathecal baclofen, selective dorsal rhizotomy, and deep brain stimulation.
Chemodenervation is one of the most commonly recommended treatments for patients with PMOA (2). When performed correctly, chemodenervation procedures (phenol, alcohol, botulinum toxins) may produce an effective, long-lasting reduction in muscle tone in the targeted muscle(s). In addition to reduced muscle tone, patients may report decreased stiffness, improved passive or active range of motion (PROM or AROM), pain, sleep, and passive or active function following chemodenervation (2).
To safely and effectively perform chemodenervation procedures requires that the clinician be familiar with the selected agent including its mechanism of action (MOA), dosing, safety profile, toxicity, risks, advantages/disadvantages, and optimal method for administering a given agent (2–4). See Table 13.1 for a full discussion of the advantages and disadvantages of the various chemodenervation agents; dose calculation, patient selection, and goal setting are covered elsewhere in this text. The focus of this chapter is a review of the most commonly used guidance techniques for localizing the targets for chemodenervation (muscles, motor points [MP], nerves, glands, or other structures). A brief review of chemodenervation agents including their MOA and reconstitution/dilution of botulinum neurotoxins (BoNTs) is provided as the properties of the individual agents influences the choice of supplementary guidance techniques (5).
CHEMODENERVATION AGENTS
Neurolytic agents. Phenol (4%–7%) and alcohol (30%–90%) have been used for decades in chemodenervation procedures and produce a long-lasting reduction in muscle tone. When injected in a near nerve location or at MP these agents diffuse into the nerve/MP causing protein denaturation and/or Wallerian degeneration of axons with resulting physical neurolysis (6–8). The resulting neurolysis blocks nerve conduction resulting in decreased signaling in the treated nerve (motor, mixed, or sensory nerves). When motor or mixed nerves are treated, neurolysis leads to a block in nerve signaling to a muscle or muscles thereby decreasing muscle tone (9). Phenol is the most commonly used agent for neurolytic procedures (for spasticity) and it is reported to have a lower incidence of neuritis than alcohol (8). In addition to its neurolytic effect, phenol also has an immediate local anesthetic effect on injection and is sometimes used for pain therapy (10).
As these potent agents are nonselective, exquisite care is required when injecting neurolytics to prevent damage to tissues adjacent to the nerve/MP target. When used to treat muscle overactivity, “pure” motor nerves are typically targeted because injection of neurolytic agents in a sensory or mixed nerve may lead to damage to sensory axons with resulting painful dysesthesias and/or sensory loss (9,11). In addition to treatment of spasticity, neurolytic agents are also used to treat pain (10).
Botulinum toxins. BoNTs are biological products of various strains (A–G) of Clostridium botulinum bacterium (gram-positive, obligate anaerobes) and recognized as one of the most potent toxins known (12). Given this fact, the widespread use of BoNTs in clinical practice for an ever-increasing number of approved and unapproved indications may seem surprising (13). Despite the potential toxicity of BoNTs these potent agents, when used by expert clinicians in very small quantities, have an excellent safety profile for treatment of muscle overactivity, pain, and neurosecretory, urological, ophthalmic, and other disorders (14,15).
TABLE 13.1
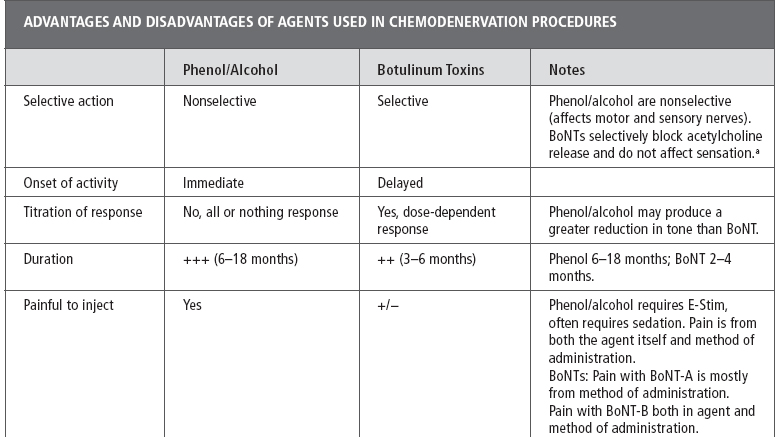
When used to treat muscle overactivity, BoNTs produce a dose-dependent decrease in muscle tone lasting several months (3). Accurately localizing a muscle (or other target tissue) is essential so as to reduce the risk of intravascular injection, weakness in untargeted muscles, injection of untargeted tissues that may be affected by BoNT (glands), or needle penetration of untargeted tissues (lung, kidney, and others [5,15]). BoNTs exert their effect in muscles by reducing the presynaptic release of acetylcholine (ACH), the neurotransmitter (NT) responsible for signaling at the neuromuscular junction (NMJ) and other cholinergic synapses. NTs are contained within synaptic vesicles, BoNTs block one or more of the suite of soluble N-ethylmaleimide sensitive receptor (SNARE) proteins that are involved in synaptic vesicle docking and the release of NTs including ACH (12–14).
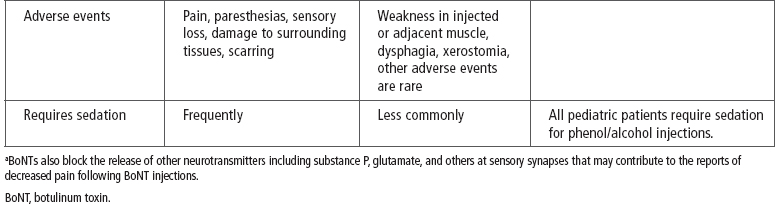
The seven strains of C. botulinum produce serologically distinct exotoxins labeled A to G. Only serotypes A and B are manufactured commercially and available for use in clinical practice (3,14). There are multiple BoNT-A serotype toxin products available worldwide but only one commercially available serotype B toxin, rimabotulinumtoxinB (RBTB; Myobloc® in the United States). The three serotype A toxins currently approved for use in the United States are onabotulinumtoxinA (OBTA; BOTOX®); abobotulinumtoxinA (ABTA; Dysport®); and incobotulinumtoxinA (IBTA; Xeomin® [14,16]; see Table 13.2). In the United States, OBTA is approved for the treatment of spasticity (upper limb muscles, adults ≥18 years of age). In other countries, OBTA and ABTA are approved for the treatment of spasticity in children and adults (16). Currently, neither IBTA nor RBTB is approved by government agencies (United States or elsewhere) for the treatment of spasticity in children or adults (14).
TABLE 13.2
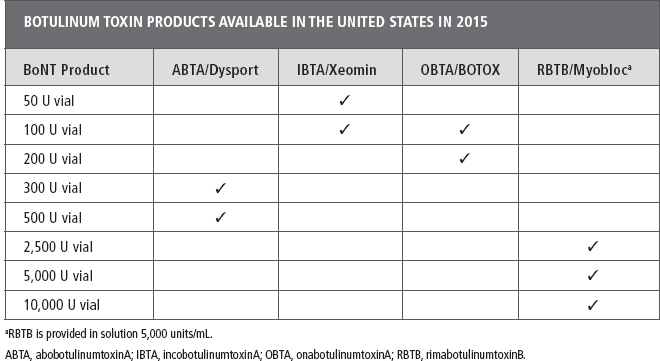
BoNTs are biological products, not drugs, therefore each commercially available BoNT product and its dosing is unique. The manufacturers of the approved BoNT products and regulatory agencies specifically state that BoNT products are not interchangeable and the dosing for each is product specific (17–20). Clinicians who use “dose conversion ratios” should do so with caution until such time that the accuracy and safety of this practice are confirmed in head-to-head clinical trials (14,16).
BoNT reconstitution. RimabotulinumtoxinB is provided in solution (5,000 units/mL) and therefore does not require reconstitution. If a more dilute preparation is desired additional preservative-free normal saline (PFNS) can be added to the vial or syringe. All of three of the BoNT-A products available in the United States require reconstitution and PFNS is recommended as the diluent (Table 13.2). For additional information on reconstitution the reader is referred to the manufacturer’s full prescribing information available with each vial of BoNT product (16–20) or to reviews of this topic (14,16,21,22).
Dilution. There are limited, conflicting data in the literature related to the effect of dilution of the various BoNT products, on their diffusion, efficacy, or adverse events following injection for treatment of muscle overactivity (21–25). Therefore, the optimal dilution for a given toxin, condition, or patient is unknown. Additional high-quality studies are required to determine the impact of dilution when using the various BoNT products in the treatment of muscle overactivity (26,27).
As noted earlier, currently, OBTA is the only BoNT product approved by the Food and Drug Administration (FDA) for the treatment of spasticity [upper limb, adults ≥18 years of age]) in the United States. The manufacturer’s recommended dilution for this condition is 100 U in 2 mL or 200 U in 4 mL PFNS (18). The published or recommended dilution for abobotulinumtoxinB when treating patients with spasticity ranges from 0.6 to 1.5 mL for a 300 U vial or 1 to 2.5 mL for a 500 U vial (14,16). There is no published information on dilution of IBTA when injecting for spasticity. The author’s practice is to dilute a 50-U vial with 0.5 or 1.0 mL of PFNS or a 100-U vial with 1.0 or 2.0 mL or to customize the dilution as described in the following section.
In clinical practice, when treating patients with spasticity, particularly severe spasticity, or when large muscle groups are targeted, many clinicians reconstitute BoNT with higher volumes of saline than described earlier. The goal is to achieve a more dilute preparation (lower number of units/mL) theoretically to enhance diffusion of BoNT within the muscle. Again, studies supporting or refuting the practice of high dilutions are limited and the results of these studies are conflicting (26–29). Higher dilutions, that is, lower concentration and/or number of units/mL, are useful when treating patients with focal dystonias or dermatologic conditions where precise dosing is often required. The higher dilution/lower number of units/mL allows for accurate dose delivery of a very small number of units of BoNT into a muscle (29,30). Lower dilutions (higher concentration or number of units/mL) are typically used when treating patients with smaller muscles, that is, younger pediatric patients. Theoretically, the goal of using a higher concentration in this situation is to prevent diffusion of the toxin into adjacent or untargeted structures (28). Additional studies are required to support or refute this theory.
When treating patients with spasticity, the author uses an alternative dilution/reconstitution scheme to the aforementioned scheme where a vial of toxin is diluted/reconstituted with a fixed volume of PFNS. The author’s practice results in a dilution that is “customized” for each muscle where the volume of saline added to the syringe is determined by muscle size, severity of the spasticity, and the functional goal (passive or active function).
The following is a description of the author’s practice:
Step 1. Initial BoNT vial reconstitution:
• OBTA 100 U vial with 1 mL or 200 U vial with 2 mL PFNS for a concentration of 100 units/mL
• ABTA 300 U in 0.6 mL or 500 U in 1 mL PFNS for a concentration of 500 units/mL
• IBTA 50 U in 0.5 mL or 100 U in 1 mL PFNS for a concentration of 100 units/mL
• BoNT-B is provided in a ready to use 5,000 units/mL solution and does not require reconstitution
Step 2. Drawing up the BoNT:
• The prescribed dose of BoNT (in units) for each muscle to be treated is drawn up into a 1 or 3 mL syringe (depending on the dose, desired dilution, and final volume)
Step 3. Customized dilution:
• Additional PFNS is then drawn up into each syringe to the desired dilution. The additional saline can be drawn by the nurse, as prescribed by the physician or the physician can draw up the additional saline
• Each syringe is labeled with the number of units and additional dilution (eg, 20 U + 0.2 mL)
Benefits of customized dilution.
• This practice allows a physician to “customize” the dilution of BoNT when treating multiple muscles which may be of different sizes, have different severity of hypertonia, and have different functional goals.
![]() For example, when treating the biceps brachii or hamstring muscles, a greater volume of PFNS is added to the syringes than would be added to syringes containing BoNT-A than the syringes for intrinsic muscles of the hand or foot.
For example, when treating the biceps brachii or hamstring muscles, a greater volume of PFNS is added to the syringes than would be added to syringes containing BoNT-A than the syringes for intrinsic muscles of the hand or foot.
• This dilution strategy reduces the confusion as the number of units/mL that may occur when multiple dilution volumes are used during reconstitution.
• This practice reduces the risk of dosing errors that may occur when reconstituting vials variable volumes of PFNS. This is particularly useful when the physician is not reconstituting or drawing up the toxin. If the toxin is always reconstituted with the same volume of PFNS, the physician can be assured of the number of units of BoNT in a given volume in a syringe (after initial reconstitution/draw up).
COMBINED NEUROLYTIC/CHEMICAL DENERVATION PROCEDURES
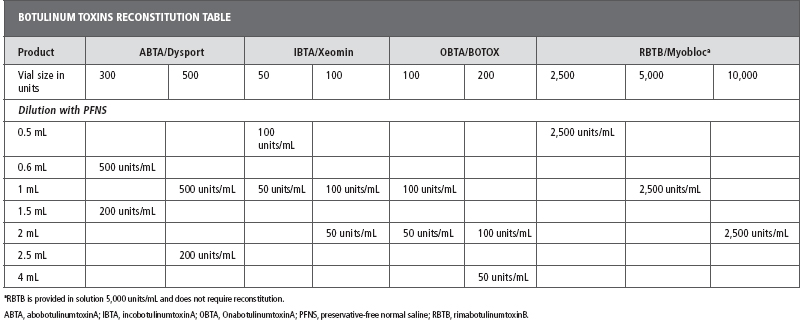
In many practices, chemodenervation with BoNTs has largely replaced the use of phenol/alcohol for neurolysis. However, each of these forms of chemoneurolysis has advantages and disadvantages and may be useful for treating hypertonia (Table 13.3 [2,11]). These two forms of chemodenervation are not mutually exclusive and some physicians may choose to combine phenol or alcohol neurolytic blocks (targeting specific nerves) with BoNT chemoneurolysis targeting other muscles. A combined chemodenervation procedure is particularly useful when treating patients with generalized spasticity where the dose of BoNT required would exceed recommended limits or where the use of phenol/alcohol nerve blocks is limited by the sensory contributions of a targeted nerve (28). For example, clinicians may choose a phenol obturator or musculocutaneous nerve block to reduce scissoring or elbow flexion-reserving BoNT to treat hypertonia in other muscles. In this situation, phenol/alcohol is used as “spare toxin” for other spastic muscles.
Treatment Planning for Chemodenervation Procedures
The first step in treatment planning and selection of guidance techniques for chemodenervation procedures is a detailed patient assessment, including a history, physical examination, and functional assessment. It is also imperative to take an interval history and reexamine the patient when he or she arrives for a scheduled chemodenervation procedure particularly with BoNT. This is needed to rule out contraindications to performing the procedure (acute illness, infection, medications, recent BoNT injections by another provider, other medical problems).
TABLE 13.3
Guidance Techniques for Chemodenervation Procedures
When performing chemodenervation procedures, precise localization of a given muscle or other target is required particularly when using nonselective neurolytic agents. Accurately targeting a structure for injection is important for many reasons, including optimizing the outcomes of the procedure, reducing the risk of tissue damage with neurolytics or required dose of BoNT, and/or minimizing potential risks/adverse events associated with these procedures (30,31).
The most appropriate guidance technique for a given procedure is determined by:
• The choice of agents for chemodenervation (neurolytic agents require electrical stimulation)
• The target structure (electromyography [EMG] is not useful for injecting glands)
• Patient-related factors (obesity, muscle atrophy, anatomic rearrangements, postsurgical changes)
• The physician’s skill or expertise with the various methods
Given the many factors involved in the choice of guidance techniques, the ideal situation is for physicians who perform chemodenervation procedures to be skilled in several techniques.
A prerequisite for performing chemodenervation procedures is a comprehensive knowledge of surface/cross-sectional and functional anatomy. In addition to knowledge of anatomy, palpation, and PROM or AROM, most physicians who perform chemodenervation procedures also make use of one or more of the available supplementary guidance techniques. The goals for using supplemental guidance are to improve the accuracy of targeting and reducing the adverse events of these procedures (30). Commonly used guidance methods include palpation; surface anatomy/reference guides; MP/end-plate targeting; EMG; electrical stimulation (E-Stim) and image localization techniques (B-mode ultrasound [US], fluoroscopy, computed tomography [CT], magnetic resonance imaging [MRI]); and/or combinations of these techniques (30–33). The following is a review of the advantages and limitations of the most commonly used guidance techniques for chemodenervation procedures (Table 13.4).
Comparative Studies of Guidance Techniques for Neurolytic Chemodenervation Procedures
Neurolytic procedures. For neurolytic procedures using either phenol or alcohol, E-Stim is the universally recommended or reported guidance technique (7–11,34,35). E-Stim combined with US is also reported to enhance the localization of peripheral nerves for lytic, diagnostic, or anesthetic nerve blocks (36–38). A literature search (PubMed, Medline) returned no published studies evaluating the use of manual needle placement or EMG guidance for neurolytic chemodenervation procedures.
TABLE 13.4
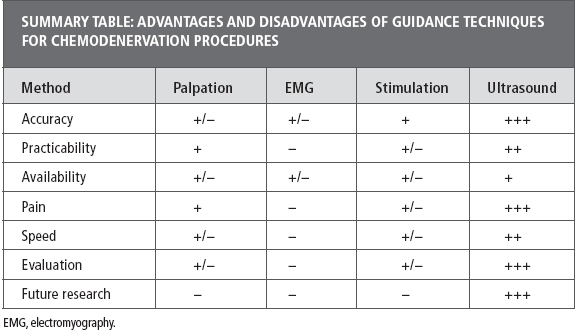
BoNT chemodenervation. A number of studies have compared the accuracy and efficacy of BoNT injections when using anatomic localization versus other guidance techniques, including EMT, E-Stim, and US.
Anatomic Guidance Methods for Chemodenervation Procedures
The importance of a physician’s knowledge of anatomy cannot be understated. This knowledge is a foundation when performing chemodenervation procedures and directs the choice of a supplemental guidance technique. No supplementary guidance replaces this knowledge of anatomy. Teaching tools that may aid clinicians in improving their knowledge of anatomy include biomechanics or anatomy textbooks, anatomy atlases (print and/or electronic), anatomy simulators, and a return to the gross anatomy lab to review dissections (39–44).
Anatomic guidance relies on surface anatomy, knowledge of cross-sectional anatomy, PROM, and/or AROM. Physicians must remember that the EMG anatomic reference guides commonly used when performing BoNT injections were not developed for this purpose. These atlases were developed to guide needle insertion for diagnostic EMG procedures (45–47). Although these texts may be useful for BoNT injections, they have limitations either when used for their original purpose or when used to guide BoNT injections. In the last several years, two anatomic atlases were published specifically for botulinum toxin and/or other chemodenervation procedures (48,49).
Although some physicians rely solely on anatomic guidance for BoNT injection in some superficial muscles the use of manual needle placement/anatomic guidance alone may not be sufficient when attempting to localize deep muscles or when complex overlapping anatomy obscures muscle identification such as in the forearm, neck, or calf (50–52).
Relying on anatomic guidance alone is not recommended for procedures using phenol or alcohol as these nonselective agents must be precisely placed in a near nerve or MP location. These procedures require the use of E-Stim or the combination of E-Stim and US (11).
Equipment and Supplies. Supplies for manual needle placement/anatomic guidance include musculoskeletal anatomy atlases, anatomic reference guides, gloves, alcohol or other skin disinfectant, gauze, adhesive bandages/plasters, single-use hypodermic needles of varying gauge (g) and length, and 1- and/or 3-mL, 5-mL syringes.
An assistant is typically required to assist the physician with patient positioning, stabilizing, and preventing movement of the limb or body part during the procedure.
After estimating the depth of the target/muscle and trajectory of the injection, the site for needle insertion and the needle for injection are selected. For superficial muscles, a 30-g ½-inch or 30-g 1-inch needle are often selected. For deeper muscles, a 26- to 27-g 1¼- to 1½-inch needle may be sufficient. For the deepest layer of muscles, a 25-g 2½- to 5-inch spinal needle may be required. Physicians are cautioned that accurately targeting deep muscles is challenging, at best, or may be impossible when relying solely on anatomic guidance.
Procedure. The patient is placed in a comfortable position, sitting, supine, or prone depending on the target structure. Once a muscle(s) or other targets are localized using a combination of surface anatomy, palpation, PROM, or AROM, the skin is disinfected using the physician’s or institution’s protocol. The needle is then inserted through the skin and advanced to the target structure. Observing movement of the needle with PROM or AROM of the muscle may confirm the location of the needle when a muscle is the target for injection. Following aspiration the BoNT is then injected. When injecting larger doses/volumes, the previous procedure is repeated at multiple injection sites.
Advantages and Limitations of Anatomic Guidance for Chemodenervation Procedures
Advantages
• All physicians take a gross anatomy course during medical school (Table 13.5).
• Anatomic reference guides are inexpensive and widely available to physicians.
• Electronic 3D references are available online.
• Anatomic simulators provide information about orientation and function of muscles.
Limitations
• A physician’s training in the gross anatomy may have been many years ago and memory of this training may have faded.
• Positioning a patient with spasticity as described in reference guides may be challenging or impossible. When not positioned as directed, the estimated/recommended site for needle insertion to target a muscle may be incorrect. Difficulty with patient positioning thus limits the accuracy of reference guides for BoNT injections in many patients.
TABLE 13.5
ADVANTAGES/LIMITATIONS OF ANATOMIC GUIDANCE FOR CHEMODENERVATION PROCEDURES | |
Anatomic Guidance/Manual Needle Placement for Chemodenervation Procedures | |
Advantages | Limitations |
All physicians receive gross anatomy training | Training in anatomy may be a distant memory |
Anatomic reference guides are widely available | Most guides are devised for use in diagnostic EMG |
Unable to position patient as illustrated in reference books. This may lead to targeting errors | |
Anatomic variations | |
Some muscles are easily localized by their surface anatomy, palpation or PROM/AROM | Many muscles cannot be palpated or localized using surface anatomy because of their deep location |
Complex overlapping anatomy obscures muscle localization | |
Obesity obscures muscle palpation and assessment of the depth and location of the muscle | |
Muscle atrophy from disuse or repeated BoNT injection is common, making it difficult to estimate depth/muscle thickness | |
Muscle rearrangement, deformities may shift location of muscle target | |
Postsurgical changes following bony or muscle surgeries may alter muscle location | |
Involuntary movements, reflex muscle activation | |
Patient cooperation or ability to follow directions | |
AROM, active range of motion; BoNT, botulinum toxin; EMG, electromyography; PROM, passive range of motion.
• Most reference guides were designed to guide needle insertion for diagnostic EMG, not injection procedures. The accuracy of relying solely on these surface anatomy and reference guides for EMG and BoNT injections has been called into question; see the following section.
• Palpation/surface anatomy: Although some superficial muscles may be easily identified by surface anatomy/palpation it may be difficult or impossible to correctly identify many muscles where:
![]() Complex overlapping obscures palpation.
Complex overlapping obscures palpation.
![]() Muscles are deeply situated limiting the use of palpation to localize or estimate muscle depth.
Muscles are deeply situated limiting the use of palpation to localize or estimate muscle depth.
![]() Obesity obscures palpation of a muscle or determining its depth.
Obesity obscures palpation of a muscle or determining its depth.
![]() Disuse atrophy or BoNT-induced atrophy alters muscle thickness limiting accuracy of estimating its depth.
Disuse atrophy or BoNT-induced atrophy alters muscle thickness limiting accuracy of estimating its depth.
![]() Anatomic rearrangements, deformities, postoperative changes following lengthening or transfers.
Anatomic rearrangements, deformities, postoperative changes following lengthening or transfers.
![]() Patient cooperation, involuntary movements, and uninhibited reflexes.
Patient cooperation, involuntary movements, and uninhibited reflexes.
Evidence for and Against Anatomic Guidance for BoNT Chemodenervation Procedures
The evidence from published studies comparing the accuracy of muscle targeting using manual needle placement (anatomic guidance) to other localization techniques (EMG, E-Stim, US) for BoNT procedures calls into question the practice of relying solely on anatomic guidance of these procedures.
Cadaver Studies
• A 2012 study evaluated the accuracy of using palpation and surface landmarks to guide injections into the gastrocnemius muscles of 30 cadavers. Following ink injection into the calf targeting the gastrocnemius (121 physicians) the muscle was dissected and the location where the injectate was placed was recorded (51). Only 43% of the injections were successfully placed within the gastrocnemius muscle. Fifty-seven percent of the injections were placed outside of the muscle, either in the soft tissue (19.8%) or deep to the gastrocnemius in the soleus muscle (37.2%).
• A 2011 blinded study compared “blind” (manual/anatomic guidance) versus US-guided placement of a wire into 14 lower limb muscles in fresh cadavers. The accuracy of targeting was checked by CT. Two clinicians (an attending with ≥10 years’ EMG experience and a resident with 6 months’ EMG experience) performed the wire insertions with the accuracy of placement checked by a third clinician (52). In the 14 tested muscles, the overall accuracy of blind wire placement was 39% (range, 0%–100%) compared to 96% (range, 50%–100%) for US-guided wire insertion.
![]() The only muscles in which blind placement was 100% accurate were the tibialis anterior and short head of the biceps femoris
The only muscles in which blind placement was 100% accurate were the tibialis anterior and short head of the biceps femoris
![]() With US the only muscle with less than 100% accuracy was the semitendinosis.
With US the only muscle with less than 100% accuracy was the semitendinosis.
![]() Clinicians may be surprised with the failure to accurately target several large superficial muscles in the lower limb using manual needle placement, including the semitendinosis, rectus femoris, and extensor hallicus longus where 0% of needle insertions were accurate.
Clinicians may be surprised with the failure to accurately target several large superficial muscles in the lower limb using manual needle placement, including the semitendinosis, rectus femoris, and extensor hallicus longus where 0% of needle insertions were accurate.
![]() Also somewhat surprising was that there was no significant difference when comparing the accuracy of the less and more experienced clinicians. The experienced clinician was only more accurate in the trajectory of needle insertion toward the target muscle.
Also somewhat surprising was that there was no significant difference when comparing the accuracy of the less and more experienced clinicians. The experienced clinician was only more accurate in the trajectory of needle insertion toward the target muscle.
• A 2003 cadaver study also evaluated the accuracy of fine wire insertions into the lower limb muscles of 10 cadavers (36 different muscles, 263 muscles in total). The wire placement was performed by three physicians with varying degrees of EMG experience using standard EMG anatomical reference guides (45,53). The accuracy of wire placement was checked by anatomical dissection by an anatomist.
![]() Although 57% of wire insertions penetrated the target muscle, the tip of the wire was only in the target muscle in 45% of insertions.
Although 57% of wire insertions penetrated the target muscle, the tip of the wire was only in the target muscle in 45% of insertions.
![]() The results of this study also revealed significant variability in the accuracy of targeting between different muscles from 100% for vastus medialis to 0% for 12 attempts in the hip flexors.
The results of this study also revealed significant variability in the accuracy of targeting between different muscles from 100% for vastus medialis to 0% for 12 attempts in the hip flexors.
![]() The proximity of the wire to other structures was also noted and in 17% of insertions the wire either penetrated or passed within 5 mm of an important structure such as a vessel or nerve.
The proximity of the wire to other structures was also noted and in 17% of insertions the wire either penetrated or passed within 5 mm of an important structure such as a vessel or nerve.
![]() The authors concluded that the accuracy of blind wire placement using EMG reference guides was variable and the development of safer strategies was recommended.
The authors concluded that the accuracy of blind wire placement using EMG reference guides was variable and the development of safer strategies was recommended.
BoNT Chemodenervation: Manual Needle Placement Versus EMG Guidance
• A 2013 randomized controlled trial (RCT) of 27 adult patients with upper motor neuron syndrome (UMNS) spasticity (brain injury, spinal cord injury) compared the effectiveness of BoNT injections in upper and lower limb muscles guided by EMG versus anatomic guidance using landmarks. Outcome measures included the Modified Ashworth Scale (MAS) for spasticity and the Modified Barthel Index as a functional outcome measure.
![]() A reduction in MAS score and improved Barthel Index score was noted in all subjects but the improvements in tone and function were greater in patients in whom BoNT injections were guided by EMG. The authors concluded that EMG was superior to using landmark-based reference guides when injecting BoNT for the treatment of spasticity (54).
A reduction in MAS score and improved Barthel Index score was noted in all subjects but the improvements in tone and function were greater in patients in whom BoNT injections were guided by EMG. The authors concluded that EMG was superior to using landmark-based reference guides when injecting BoNT for the treatment of spasticity (54).
• In a 2003 study of adult patients with focal hand dystonia the authors reported greater efficacy of BoNT injections guided with EMG compared to manual needle placements (55). The authors concluded that EMG injections were superior to manual/anatomically guided placement for focal hand dystonia.
BoNT Chemodenervation: Manual Needle Placement Versus Guidance by E-Stim
• A 2009 study of BoNT injections in children with cerebral palsy (CP; hemiplegia or diplegia) compared the efficacy of BoNT procedures guided by palpation versus E-Stim. At 12 weeks, patients whose injections were guided by E-Stim had a statistically greater reduction in MAS and Composite Spasticity Scale scores, improved PROM and Gross Motor Function Measure scores when compared to those whose injections were performed using manual guidance alone (56).
• A 2005 study of BoNT injections for spasticity in upper and lower limbs in 226 children with CP evaluated the accuracy of manual needle placement (1,376 needle insertions, three experience injectors) checked by E-Stim (57). Manual needle insertion site and depth were determined by palpation, surface anatomy, PROM (if possible), and limb size. After the clinician manually placed the needle, the accuracy of the needle position was checked by using E-Stim, observing for muscle twitch pattern on stimulation.
![]() The authors reported the accuracy of manual placement ranged from 11% to 78% (gastroc-soleus, 78%; hip adductors, 67%; biceps brachii, 62%; medial hamstrings, 46%; adductor pollicis, 35%; pronator teres, 22%; flexor carpi ulnaris (FCU), 16%; flexor carpi radialis (FCR), 13%; tibialis posterior, 11%).
The authors reported the accuracy of manual placement ranged from 11% to 78% (gastroc-soleus, 78%; hip adductors, 67%; biceps brachii, 62%; medial hamstrings, 46%; adductor pollicis, 35%; pronator teres, 22%; flexor carpi ulnaris (FCU), 16%; flexor carpi radialis (FCR), 13%; tibialis posterior, 11%).
![]() The authors concluded that for the tested muscles manual placement was adequate only in the gastrocnemius. They also postulated that inaccurate muscle targeting could be responsible, at least in part, for a lack of or insufficient clinical response following BoNT injections in children with CP.
The authors concluded that for the tested muscles manual placement was adequate only in the gastrocnemius. They also postulated that inaccurate muscle targeting could be responsible, at least in part, for a lack of or insufficient clinical response following BoNT injections in children with CP.
BoNT Chemodenervation: Manual Needle Placement Versus US or E-Stim
• A 2012 RCT of 49 adult patients with poststroke spasticity (PSS) evaluated the efficacy of BoNT injections in the gastrocnemius (58). The study compared the efficacy of BoNT injections using three localization techniques (manual, E-Stim, US) using a fixed dose and dilution protocol (OBTA 100 U medial head, 100 U lateral, dilution 100 U reconstituted with 2 mL PFNS).
![]() The authors reported a greater reduction in MAS scores at 4 weeks when injections were guided with US compared to injections guided by manual needle placement.
The authors reported a greater reduction in MAS scores at 4 weeks when injections were guided with US compared to injections guided by manual needle placement.
![]() The improvement in PROM was greater in the US-guided group compared to the E-Stim and manual guidance groups.
The improvement in PROM was greater in the US-guided group compared to the E-Stim and manual guidance groups.
![]() There was no statistically significant difference in the Tardieu Scale score among the three groups. The authors concluded that the US guidance provided greater reduction in MAS and improvement in PROM than manual and/or E-Stim guidance.
There was no statistically significant difference in the Tardieu Scale score among the three groups. The authors concluded that the US guidance provided greater reduction in MAS and improvement in PROM than manual and/or E-Stim guidance.
• A 2009 study of 39 children with spasticity from CP compared the accuracy of blind/manual needle placement in the gastrocnemius muscles checked by US by a blinded clinician (59). The overall accuracy of blind placement in the gastrocnemius was 78.7%. However, the accuracy of needle placement in the thinner lateral gastrocnemius muscle was only 64% for the group and 46% in younger patients. This was compared to an overall accuracy of 93% in medial gastrocnemius (87% in younger patients).
![]() The authors concluded that a supplementary targeting technique should be considered for lateral gastrocnemius injections in patients with CP (all ages) and for the medial gastrocnemius in younger patients.
The authors concluded that a supplementary targeting technique should be considered for lateral gastrocnemius injections in patients with CP (all ages) and for the medial gastrocnemius in younger patients.
• Another 2009 study of 54 children with spasticity from CP assessed the impact of several variables on the effectiveness of BoNT injections in lower limb muscles (60). Variables included patient age, dose, dilution, muscles injected, and guidance techniques. BoNT injections were guided by manual needle placement in 44% of patients and by US in 56% of patients.
![]() The authors reported a greater efficacy when injections were guided by US, patients were younger than 6 years or older than 12 years, the muscles injected were hamstrings or gastrocnemii, and dose/muscle was greater than 0.8 units/kg (OBTA). Dilution had no effect on efficacy. The authors concluded that the results of this study confirmed the usefulness of US guidance for BoNT injections in lower limb muscles.
The authors reported a greater efficacy when injections were guided by US, patients were younger than 6 years or older than 12 years, the muscles injected were hamstrings or gastrocnemii, and dose/muscle was greater than 0.8 units/kg (OBTA). Dilution had no effect on efficacy. The authors concluded that the results of this study confirmed the usefulness of US guidance for BoNT injections in lower limb muscles.
• A 2010 study of forearm flexor muscles in patients with spasticity assessed muscle and/or muscle fascicle localization using anatomic reference guides compared to the location as checked by US (50). There were significant differences between the position of the muscle or muscle fascicles estimated by the manual techniques when compared to US for the FCR, flexor polices longus (FPL) and for the fascicles of the flexor digitorum superficialis (FDS).
Conclusions
Although a thorough knowledge of surface and functional anatomy is a prerequisite for physicians who perform BoNT injections, relying solely on anatomical guidance and reference guides may not be the optimal method to guide BoNT injections. Although many clinicians who use blind/manual needle placement to guide BoNT injections report good results with this technique, no study to date has shown the superiority of this technique when compared to EMG, E-Stim, or US. Because of the limitations of manual needle placement many if not most clinicians use one or more add-on guidance technique when performing BoNT chemodenervation procedures (Table 13.5).
Motor End-Plate Targeting for BoNT Injections
BoNT exerts its action at the NMJ by avidly binding to presynaptic receptors and blocking the release of ACH. This knowledge has led clinicians and researchers to postulate and investigate the influence of motor end plate (MoEP) targeting on BoNT uptake or efficacy (61–64). Using published information on the location of MoEPs in various skeletal muscles, physicians can incorporate MoEP targeting into whatever guidance technique is used for BoNT chemodenervation procedures.
Published Studies on the Location and Distribution of MoEPs/MPs
The location of MP points or end-plate zones in mammalian muscles has been studied in animal models and in humans using histochemical staining and electrophysiological methods (64–71)
• In 1958, Coers described three types or arrangements of MoEP in human muscles: (a) muscles having a single innervation band, (b) muscles with multiple innervation bands, and (c) muscles where the innervation bands were scattered throughout the muscle (71).
• In 1959, Christensson published data on MoEP topography, distribution, and pattern in stillborn infants. She reported that in unipennate muscles MoEP were distributed in a single transverse band at the midpoint of the muscle and that in the bipennate gastrocnemius muscle, the MoEPs were arranged in a concave band (70). More recently, an inverted V or concave band arrangement of MoEP has also been reported in another bipennate muscle, the biceps brachii, by another group (72).
• Alternately, in 2005, Kim et al reported that the MoEPs of the gastrocnemius and soleus are distributed diffusely along the length of the muscle (73).
• Van Campenhout et al reported on the location and distribution of MoEP in the psoas muscle of adult cadavers (74). The authors reported that the motor MoEPs were distributed along a zone between 30.83% and 70.25% of the distance from T12 to the inguinal ligament. Therefore, the majority of the MoEPs were proximal to the sacral promontory.
For additional details on MoEP localization, the reader is referred to several anatomical studies and review articles published in the last decade that detail the location and distribution of MoEPs in upper and lower limb muscles (61,64–74).
Evidence Supporting MoEP Targeting
Although MoEP targeting has been suggested or recommended for BoNT injections for decades (61,63,68), there are limited data from controlled trials evaluating the superiority of MoEP targeting alone or in combination with other localization techniques (64,71–73).
• There are limited data from animal and human studies that with MoEP targeting a reduced dose of BoNT is required to reduce muscle tone and/or the compound motor action potential (CMAP [65–67,72,75]). This suggests that MoEP targeting may reduce the required dose of BoNT that is required for efficacy and that this reduced dose may potentially reduce dose-related adverse events (65,66,72).
• A 2014 double-blind RCT (DB-RCT) compared the efficacy of a fixed BoNT dose placed within the MoEP zone (suggested by anatomical studies) at 2/10 and 3/10 of calf length to injections below the mid-belly of the muscle. Although both groups improved, there were no statistical differences between the two groups in either clinical or electrophysiological measures (39).
• In 2009, Gracies et al published the results of a DB-RCT comparing a fixed dose of OBTA using either standard volume dilution (100 U in 1 mL) using MoEP targeting and injection in four quadrants of the muscle without targeting the MoEP (using the previously noted dilution) and four quadrant injections using high volume dilution (100 U in 5 mL). The authors reported a greater benefit with injections using MoEP targeting than those with non-MoEP targeting or injection with high volume dilution (76).
• A 2011 article reviewed the evidence on location of MoEP in lower limb muscles and recommended an optimal injection zone for the gastrocnemius, soleus, tibialis posterior, semitendinosis, semimembranosus, biceps femoris, gracilis, rectus femoris adductors longus, brevis, magus, and psoas muscles (61). This article was limited to a review of MoEP locations and did not provide data on efficacy of targeting MoEP compared to other techniques.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







