Fig. 3.1
Associations between the presence of T2DM and vertebral fractures. The relative risk of vertebral fractures in T2DM patients is significantly higher than that in nondiabetic subjects in both genders after adjustment for age, BMI, and L2–4 BMD (From Ref. [23])
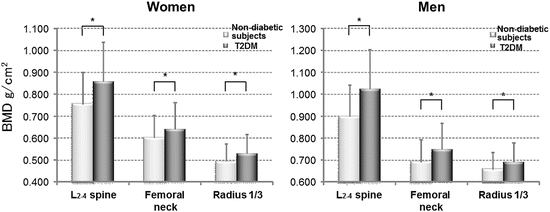
Fig. 3.2
Comparison of BMD between control subjects and patients with T2DM. BMD values at any site in T2DM patients are significantly higher than those of nondiabetic subjects in both genders. * P < 0.05 (From Ref. [23])
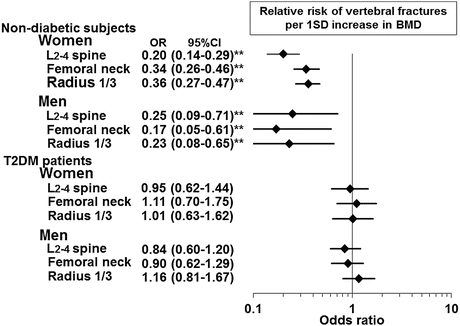
Fig. 3.3
Association between BMD and vertebral fractures in nondiabetic subjects and patients with T2DM. In contrast to nondiabetic subjects, the association between BMD and risk of vertebral fracture is not observed in T2DM patients. ** P < 0.01 (From Ref. [23])
Osteoporosis is defined as “a skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture,” and bone strength consists of “BMD” and “bone quality” [26] (Fig. 3.4). Estimation of bone strength by BMD is difficult for diabetic subjects; therefore, poor bone quality is the most suitable and explicable cause for elevated fracture risk in this population and may be a diabetes-specific mechanism for bone fragility.
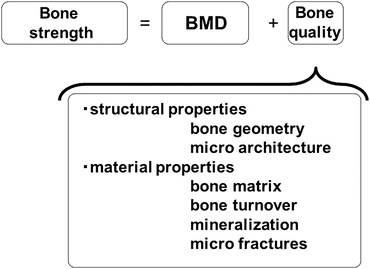

Fig. 3.4
Definition of bone strength. Bone strength consists of “BMD” and “bone quality,” the latter includes structural properties and material properties (From Ref. [26])
3.3 Mechanism of Decreased Bone Quality in Diabetic Patients
Bone quality is divided into material properties and geometrical properties (Fig. 3.4): the former reflects the physical characteristics of the bone, and the latter indicates the morphological characteristics of the bone. Material properties include the “bone matrix” composed mainly of collagen; “bone turnover,” which shows the metabolic rate at which old bone tissue is absorbed and replaced by new bone tissue; “mineralization” of the bone tissue; and “microfractures,” which are cracks confirmed using electron microscopy. The geometrical properties include “bone geometry” of the cortical bone on a macroscopic level and “microarchitecture” of the trabecular bone on a microscopic level [26]. Specific factors which are clinically associated with the risk of fracture independent of BMD are etiologic causes of deterioration of bone quality and may serve as a powerful clue for elucidating the pathology of bone fragility in diabetic patients.
3.3.1 Deterioration of the Bone Matrix and Bone Fragility
Type I collagen is the main constituent protein of the bone matrix; the formation of cross-links between neighboring collagen molecules by enzymatic reaction changes it into stabilized collagen fiber, which determines the mechanical strength of the bone tissue. Pentosidine, one of the advanced glycation end products (AGEs) which is known to be increased in diabetic patients, is composed of lysine and arginine cross-linked by a pentose. Saito et al. showed that pentosidine was increased in bone collagen content just before the onset of diabetes in spontaneously diabetic rats and that bone strength measured by three-point bending fixture test in the diabetic group was significantly decreased compared to that in the control group [27, 28]. Indeed, the negative correlation between bone strength and bone pentosidine content has been confirmed in nondiabetic patients with hip fracture [29, 30]. These findings suggest that hyperglycemic condition pathologically promotes the excessive glycosylation of bone collagen that is assumed to form cross-linking between collagen fibers by a non-enzymatic mechanism. This change in material properties of bone collagen may be a plausible cause for poor bone quality, that is, deteriorated bone strength that cannot be assessed by BMD.
Bone content of pentosidine is significantly and positively correlated with its serum concentration [31]. Clinical studies showed that increased serum and urinary pentosidine concentrations were related to an increased risk of vertebral as well as clinical fractures independent of BMD in T2DM patients [32, 33] (Fig. 3.5). These observations indirectly suggested that advanced glycation of bone collagen in patients with diabetes also deteriorates material property of bone tissue. A microindentation method recently reported directly measures material properties of bone based on the depth of the dimple impacted by the testing probe on the tibia [34]. This method revealed that bone material strength of T2DM women was significantly lower than that of age-matched nondiabetic postmenopausal women [35], which directly revealed the presence of poor bone quality caused by deteriorated material property of the bone tissue in T2DM patients.
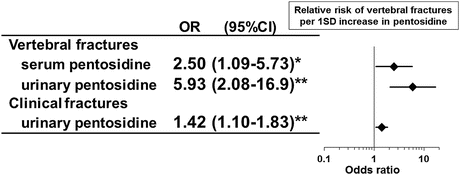

Fig. 3.5
The association between serum or urinary pentosidine levels and vertebral as well as clinical fractures in T2DM patients. Increased serum and urinary pentosidine concentrations were related to an increased risk of vertebral as well as clinical fractures independent of BMD after adjustment for multiple variables at least including age, BMI, HbA1c, renal function, and BMD. * P < 0.05, ** P < 0.01
3.3.2 Bone Turnover and Bone Fragility
The collagen products as well as the factors for mineralization, which are secreted from osteoblast during its maturity, and the collagen degradation products derived from bone tissue resorption by osteoclasts are indices for bone turnover. Parathyroid hormone (PTH) and the bone formation as well as resorption markers in T2DM patients are significantly lower than those in nondiabetic subjects (Fig. 3.6), indicating that these patients possess suppressed bone turnover [36, 37]. The subgroup with relatively lower bone formation in addition to lower PTH levels has a higher risk of vertebral fracture independent of BMD compared to the subgroup with relatively higher these values. This finding suggests that low bone turnover accompanied with decreased bone formation causes deterioration of the bone quality. Decreased ratio of osteocalcin (OC) to bone alkaline phosphatase (BAP), which is secreted proteins, the former from mature osteoblasts or osteocytes and the latter from pre-osteoblasts, is associated with increased risk of vertebral fracture independent of BMD [38], suggesting that maturation disorders of osteoblast may be involved in the poor bone quality.
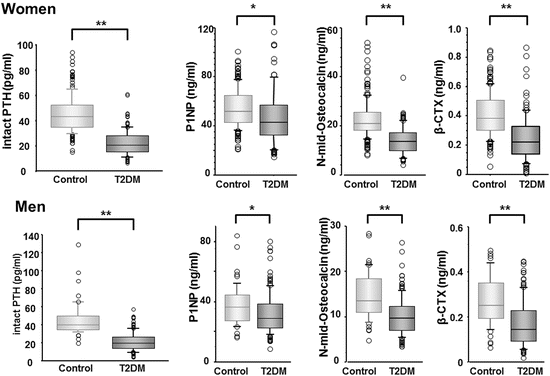

Fig. 3.6
Comparison of intact PTH and bone metabolic markers values between controls and T2DM patients. Parathyroid hormone (PTH) and the bone formation as well as resorption markers in T2DM patients are significantly lower than those in nondiabetic subjects. *, P < 0.01; **, P < 0.001 (From Ref. [36])
In addition, various factors for regulating bone turnover are reported to be involved in a risk of fracture independent of BMD. Insulin-like growth factor-1 (IGF-1), which is abundantly present in both circulating blood and bone matrix, is an important local factor for promoting the proliferation and differentiation of osteoblasts [39, 40]. IGF-1 activates the canonical Wnt/β-catenin pathway by increasing the concentration of intracellular β-catenin via promoting the degradation of glycogen synthase kinase-3 (GSK-3) after binding to the insulin receptor substrate (IRS-1) [41]. The serum IGF-1 level in female T2DM patients is lower than that in nondiabetic subjects [42], which is related to an increased risk of vertebral fractures independent of BMD [42, 43].
Sclerostin is a protein secreted by osteocytes that binds to the osteoblast LDL receptor-related proteins 5 and 6 (LRP5/6) and suppresses the canonical Wnt/β-catenin pathway by inhibiting receptor complex formation. Elevated sclerostin level is significantly associated with an increased risk of vertebral fractures, independent of BMD and bone turnover [42, 44].
The receptor for AGEs, which is presented on specific cell surface, recognizes AGEs as ligands [45] and is involved in the progression of diabetic complications such as diabetic nephropathy [46]. The studies on osteoblast derived from mice showed that hyperglycemia and AGEs suppress osteoblastic differentiation and mineralization accompanied with enhanced expression of RAGE [47–49] and that BMD was decreased in RAGE-deficient animals [50], suggesting that the AGEs-RAGE axis is involved in bone formation. Splicing variant of this receptor lacking a membrane-spanning portion is known as endogenous secretory RAGE (esRAGE), which acts as “decoy receptor” inhibiting RAGE on the cell membrane from binding to AGEs outside the cell [51] (Fig. 3.7). Irrespective of sex, the conditions of low esRAGE values and relatively low esRAGE values compared to AGEs are associated with an increased risk of vertebral fractures that are independent of BMD [52]. These findings suggest that AGEs are associated not only with glycation-induced physical changes to bone tissue but also with the pathogenesis of decreased bone quality through the biological effects mediated by RAGE.
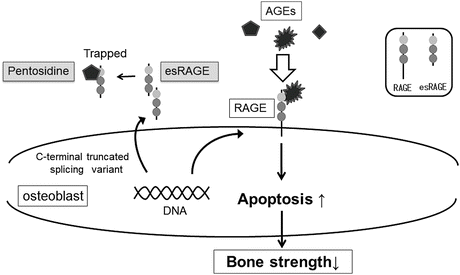

Fig. 3.7
The relationship between advanced glycation end-products (AGEs) and RAGE as well as esRAGE. Splicing variant of receptor for AGEs (RAGE) lacking a membrane-spanning portion is known as endogenous secretory RAGE (esRAGE), which acts as “decoy receptor” inhibiting RAGE on the cell membrane from binding to AGEs outside the cell. AGEs advanced glycation end-products, RAGE receptor for AGEs, esRAGE endogenous secretory RAGE
These findings indicate that inhibitory factors for bone formation are associated with fracture risk independent of BMD. Under the low bone turnover coupled with low bone formation, hyperglycosylated bone collagen or microfractures may accumulate in the bone matrix. As a result of these metabolic disorders, bone fragility may increase due to deterioration of bone material properties.
3.3.3 Structural Properties of Bone and Bone Fragility
Bone strength of cylindrical bones such as the extremities and femoral neck rises as the external diameter and cortical bone thickness increase. The distal one-third of the radius in men with T2DM is narrower than that in nondiabetic patients [53]. In addition, diabetic patients with higher or equal HbA1c values of 7.5 % have narrower external diameters of the femoral neck and a higher hazard ratio for fractures [54]. Recent progress in diagnostic imaging technology, high-resolution peripheral quantitative computed tomography (HR-pQCT), has shown that T2DM patients with fractures have significantly advanced cortical porosity at the radius as well as the tibia compared to those without fracture [55, 56] and revealed that the bone strength of these patients calculated by bone geometry of the cortical bone is decreased. In addition, trabecular bone score (TBS), which reflects finesses of cancellous bone structure, is significantly lower in T2DM patients with major fractures than that without fracture [57], indicating that exacerbated microarchitecture also affects bone strength. Because diminished bone strength caused by bone morphology is not reflected by BMD, deterioration of structural bone quality is also considered as one of the crucial pathogeneses of increased bone fragility in diabetic patients.
3.4 Diabetic Therapy and Bone Fragility
Achieving favorable control of blood glucose may be effective in preventing fractures through keeping appropriate bone turnover, because improvement of blood glucose recovers decreased marker levels of bone formation [58, 59]. However, some antidiabetic agents have been reported to influence bone turnover negatively. Large-scale surveys indicated that insulin secretagogue and metformin were not associated with the risk of fracture, rather reduce it [60–63]. Several studies showed that patients with insulin therapy have a higher risk of fractures than those with other antidiabetic therapies [19, 60–65], which is considered as an adverse effect of insulin deficiency or poor blood glucose control. Glucose-dependent insulinotropic polypeptide (GIP) [66, 67] and glucagon-like peptide-1 (GLP-1) [68, 69], which are called incretins, have been reported to increase bone mass in genetically modified animals. However, unlike the result from the first meta-analysis among short-term administration of various dipeptidyl peptidase-4 (DPP-4) inhibitors [70], a recent clinical study of long-term outcomes after treatment with certain DPP-4 inhibitors has been reported to show an increased risk of fractures [71]. In addition, results of GLP-1 treatment on increase in BMD in animal studies are inconsistent with those in clinical studies [72]. To date, established conclusion that incretin treatment decreases the risk of fractures in diabetic patients has not been obtained. Treatment with dapagliflozin, one of the sodium-glucose co-transporter 2 (SGLT2) inhibitors, did not significantly decrease BMD compared to that with metformin as control drug during 2 years’ administration [73]. In contrast, thiazolidine is known to suppress differentiation of undifferentiated mesenchymal cells into osteoblasts via activation of peroxisome proliferator-activated receptor gamma (PPARγ), and it results in decreasing bone formation. Meta-analyses have shown that the patients treated with thiazolidine have a significantly decreased BMD at lumbar vertebrae or femoral neck, compared with those treated with other hypoglycemic agents, irrespective of sex [74, 75], and that their fracture risk of hip, extremities, and all of osteoporotic fractures is significantly higher than those treated with non-thiazolidine agents [74, 76, 77].
3.4.1 Therapeutic Effect of Osteoporosis Drugs for Diabetic Patients
None of the clinical studies have clarified whether osteoporosis drugs can prevent fractures in diabetic patients. When considering the pathological state of osteoporosis in diabetic patients, bone fragility in patients with diabetes may be rescued by improvement of bone formation or material properties. In the subanalysis of the MORE study, which demonstrated the preventive effect of raloxifene on vertebral fracture in postmenopausal osteoporotic women, the risk of vertebral fracture in the subgroup with diabetes at the baseline is lower than that with the nondiabetic subgroup [78]. When a nondiabetic animal model which experimentally induced pentosidine was treated with raloxifene, bone strength recovers presumably through decreasing bone content of pentosidine [79]; therefore, raloxifene administration to diabetic patients is expected to improve the material properties of the bone matrix and prevent fractures. On the other hand, the risk of fracture in diabetic patients also increases along with decrease in BMD [16]; therefore, agents which are capable of increasing BMD may be useful in preventing fractures. Teriparatide, the only current agent promoting bone formation, decreased bone pentosidine content in addition to increasing BMD in nondiabetic animal model [80]. Teriparatide may be useful as a treatment for osteoporosis in diabetic patients, because of its pleiotropic effects, including recovery of impaired bone turnover in diabetic patients and improvement of bone matrix quality. On the other hand, bisphosphonates, which suppress bone absorption, increase BMD in T2DM patients whose bone turnover decreased, similarly to nondiabetic subjects [81], suggesting that these drugs may particularly possess advantage for preventing fracture in the diabetic patients with decreased BMD.
3.5 Conclusion
Statistical confirmation of increased risk of fracture in patients with diabetes reminds us that diabetes is one of the crucial underlying illnesses for secondary osteoporosis. This increased risk of fracture may be affected by bone extrinsic factor, such as the increased risk of falls associated with diabetic complications or treatment. However, decreased bone quality may be a major cause of bone fragility in diabetic patients, because of the increased risk of atraumatic fractures such as vertebral fractures and the presence of more excessively increased risk of fracture than expected by BMD. The bone fragility observed in diabetic patients is caused by unique pathogenesis in diabetes, suggesting that osteoporosis in diabetic patients may be one of the diabetic complications and that specific diagnostic criteria for this osteoporosis are required. Further researches are needed to develop easy tools for assessment of bone strength of diabetic patients such as bone quality markers closely related to bone fragility.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







