Chapter 90 Fatty Acid Metabolism
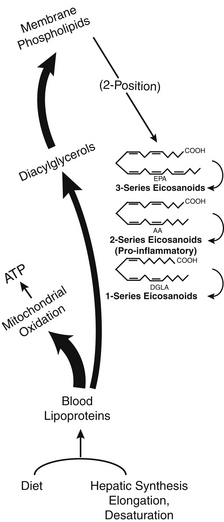
The essential hormone function is mediated by some special fatty acids that affect energy flow, cell division, immune responses, and many other body controls. These critical fatty acids are used to make powerful tissue-specific compounds called eicosanoids. Figure 90-1 illustrates the various roles of fatty acids. This chapter discusses more about these functions after some basic fatty acid relationships are explained.
 Fatty Acid Structure and Metabolism
Fatty Acid Structure and Metabolism
About 30 structural isomers and homologs of fatty acids occur in human tissues. They are named in various ways, including those with Latin prefixes such as penta– and hexa– for the number of carbon atoms. These are the chemical names approved by the International Union of Pure and Applied Chemists. Many also have common names. Because common names are in widest use among clinicians, they are used here. Table 90-1 provides a lexicon of fatty acid terms.
TABLE 90-1 Lexicon of Fatty Acid Terms
| TERM OR ABBREVIATION | DEFINITION OR EXPLANATION |
|---|---|
| AA | Arachidonic acid |
| ALA | Alpha-linolenic acid |
| Alpha-linolenic acid | Delta 9,12,15 octadecatrienoic acid; 18:3 ω-3 |
| Arachidonic acid | Delta 5,8,11,14 eicosatetraenoic acid; 20:4 ω-6 |
| Beta-oxidation | The mitochondrial metabolic pathway whereby fatty acids are converted into acetyl CoA, which enters the citric acid cycle to ultimately yield adenosine triphosphate. The carbon atoms of the fatty acid are oxidized to carbon dioxide. |
| Carnitine | The molecule to which fatty acids are attached in the process of their enzyme-mediated entry into the mitochondrial matrix. This enzyme is inhibited by malonyl CoA formed during fatty acid synthesis from carbohydrates. |
| Cerebronic acid | The 2-hydroxy derivative of lignoceric acid (24:0); found in glycosphingolipids in the brain |
| Cervonic acid | Another name for docosahexaenoic acid |
| cis | Geometrical isomer in which two groups are on the same side of a double bond |
| Clupanodonic acid | Another name for δ-7,10,13,16,19-docosapentaenoic acid (DPA3), an ω-3 series, long-chain, highly unsaturated fatty acid; found in fish oils and phospholipids in the brain |
| Delta | Used to describe the position of double bonds relative to the carboxyl end of a fatty acid |
| Desaturase | Tightly bound to the endoplasmic reticulum membrane, this enzyme, in association with cytochrome b5 and cytochrome b5 reductase, uses reduced nicotinamide adenine dinucleotide and oxygen to introduce double bonds into fatty acids. There are at least four separate desaturases, named according to the position of inserted double bonds. Delta-9-desaturase, δ-6-desaturase, and δ-5(4) desaturase act on fatty acetyl CoA thioesters, always inserting double bonds between the thioester bond (carboxylate group) and the double bond closest to it, leaving a three-carbon gap. Activities of these enzymes fluctuate according to dietary fat intake to maintain optimal fluidity state of the membrane lipids. Their concentrations decrease in starvation and increase greatly on refeeding carbohydrates. They are suppressed when dietary unsaturated fatty acid intake (including trans isomers) is high. |
| DGLA | Dihomogammalinolenic acid |
| Dihomogammalinolenic acid | Delta 8,11,4 eicosatrienoic acid; 20:3 ω-6 |
| DHA | Delta 4,7,10,13,16,19 docosahexaenoic acid; 22:6; an ω-3 series long-chain, highly unsaturated fatty acid; found in fish oils and the phospholipids in the brain (also known as cervonic acid) |
| Dienoic | Contains two double bonds |
| EFA | Essential fatty acid |
| Eicosanoid | A product of the specific, enzyme-directed oxidation of polyunsaturated fatty acids containing 20 (eicosa)carbons. This term encompasses the prostaglandins, thromboxanes, and leukotrienes. |
| Eicosapentaenoic acid | Delta 5,8,11,14,17 eicosapentaenoic acid; 20:5 ω-3; one of the most abundant fatty acids in fish oils |
| Elongase | An enzyme that adds 2-carbon units (acetate) to the carboxyl end of an existing saturated or unsaturated fatty acid. Mitochondrial form uses acetyl CoA, whereas endoplasmic form has malonyl CoA as substrate. |
| EPA | Eicosapentaenoic acid |
| Gamma linolenic acid | Delta 6,9,12 octadecatrienoic acid; 18:3 ω-6 |
| GLA | Gamma-linolenic acid |
| HUFA | Highly unsaturated fatty acid; generally having five or six double bonds |
| LA | Linoleic acid |
| Lauric acid | Tetradecanoic acid; C12:O; first isolated and identified from the laurel plant |
| LCP | Long-chain polyunsaturated fatty acid |
| Linoleic acid | Delta 9,12 octadecadienoic acid; 18:2 ω-6 |
| Meads acid | Delta 5,8,11 eicosatrienoic acid; 20:3 ω-9. This compound is not normally produced in appreciable amounts due to the preferential loading of the desaturase enzymes with the more strongly binding essential fatty acids and their metabolic products. It accumulates, however, in essential fatty acid deficiency, making it a marker for this condition. |
| Monoenoic | Containing one double bond |
| MUFA | Monounsaturated fatty acid |
| Omega | Used to describe the position of double bonds relative to the methyl end of a fatty acid |
| P + M/S ratio | Polyunsaturated + monounsaturated to saturated fatty acid ratio |
| P/S ratio | Polyunsaturated-to-saturated fatty acid ratio |
| Phospholipase A2 | An enzyme that catalyzes the hydrolysis of the fatty acid ester from position 2 of phosphoglycerides. Dependent on calcium, this enzyme is responsive to intracellular calcium, calmodulin, etc. It releases primarily polyenoic fatty acids (arachidonic acid, etc.) from membranes for eicosanoid synthesis. |
| PUFA | Polyunsaturated fatty acid |
| Phosphatide | Diacylglycerol phosphate, to which various groups may be attached through phosphoester linkage; the principal components of cell membranes |
| Polyenoic | Containing two or more double bonds |
| Saturated fatty acid | A fatty acid in which all of the carbon atoms except for the carboxyl carbon are fully hydrogenated as −CH2− (and −CH3) |
| Stearidonic acid | Delta 6,9,12,15 octadecatetraenoic acid; 18:4 ω-3; a product of elongation of ALA and a precursor to EPA. A good source is black currant seed oil. |
| Timnodonic acid | Another name for EPA or 20:4 ω-6 |
| Trans | Geometrical isomer in which two groups are on opposite sides of a double bond |
| Unsaturated fatty acid | A fatty acid in which two or more adjacent pairs of carbon atoms are lacking hydrogen atoms, having instead an additional carbon–carbon bond or double bond |
| δ | The Greek letter delta |
| ω | The Greek letter omega |
CoA, coenzyme A.
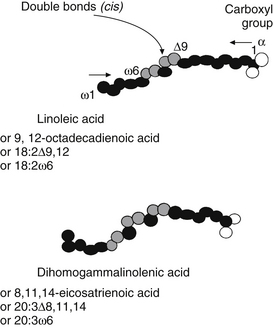
Three major families of UFAs are found in human tissues: the ω-9, ω-6, and ω-3 UFAs (or n-9, n-6, and n-3). The ω-6 and ω-3 PUFAs are defined by the position of the double bond closest to the terminal methyl group of the fatty acid molecule. In the ω-6 family, the first double bond occurs between the sixth and seventh carbons from the methyl group end of the molecule, whereas in the ω-3 family, the first double bond occurs between the third and fourth carbons (Figure 90-2 provides naming conventions).
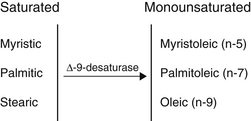
The geometry of desaturase enzymes will not allow insertion farther than nine carbons (δ-9) from the carboxyl group. Thus, linoleic acid (LA; δ-9, 12) cannot be synthesized in humans. Fatty acids of various chain lengths can act as desaturase substrates, as well as those that already possess double bonds at other positions. Figure 90-3 shows the products of the δ-9 desaturase acting on three saturated fatty acids.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree