Exertional leg pain is a common condition seen in runners and the general population. Given the broad differential diagnosis of this complaint, this article focuses on the incidence, anatomy, pathophysiology, clinical presentation, diagnostic evaluation, and management of common causes that include medial tibial stress syndrome, tibial bone stress injury, chronic exertional compartment syndrome, arterial endofibrosis, popliteal artery entrapment syndrome, and entrapment of the common peroneal, superficial peroneal, and saphenous nerves. Successful diagnosis of these conditions hinges on performing a thorough history and physical examination followed by proper diagnostic testing and appropriate management.
Key points
- •
A detailed history, physical examination, and evidence-based approach to diagnostic testing are essential in the evaluation and management of exertional leg pain.
- •
The clinician should be aware of the possibility of more than 1 diagnosis to explain complex presentations of exertional leg pain.
- •
The physical examination is often normal at rest, and provocative maneuvers or exertional activities are often required to precipitate examination findings.
Introduction
Exertional leg pain (ELP) is defined as pain distal to the knee and proximal to the talocrural joint that is associated with exertion.
The incidence of ELP in runners varies in the literature, with one retrospective study of 2002 running injuries reporting a rate of 12.8%, and another reporting that 82.4% of cross-country athletes had a history of ELP. A further study noted that running more than 40 miles per week was associated with patients presenting with ELP.
ELP is commonly categorized as having a musculoskeletal, vascular, or neurologic origin. This article focuses on medial tibial stress syndrome (MTSS), tibial bone stress injury (TBSI), chronic exertional compartment syndrome (CECS), arterial endofibrosis, popliteal artery entrapment syndrome (PAES), and entrapment neuropathies.
Introduction
Exertional leg pain (ELP) is defined as pain distal to the knee and proximal to the talocrural joint that is associated with exertion.
The incidence of ELP in runners varies in the literature, with one retrospective study of 2002 running injuries reporting a rate of 12.8%, and another reporting that 82.4% of cross-country athletes had a history of ELP. A further study noted that running more than 40 miles per week was associated with patients presenting with ELP.
ELP is commonly categorized as having a musculoskeletal, vascular, or neurologic origin. This article focuses on medial tibial stress syndrome (MTSS), tibial bone stress injury (TBSI), chronic exertional compartment syndrome (CECS), arterial endofibrosis, popliteal artery entrapment syndrome (PAES), and entrapment neuropathies.
Medial tibial stress syndrome
The incidence of MTSS in runners is between 13.6% and 20.0%. Other names for this condition include shin soreness, tibial stress syndrome, medial tibial syndrome, shin splints syndrome, and shin splints. The most commonly accepted definition of MTSS is pain along the posteromedial border of the tibia that occurs during exercise, excluding pain from ischemic origin or signs of stress fracture.
MTSS affects the posteromedial tibia, most commonly in the middle or distal third. The exact mechanism of injury is unclear, but studies show that patients with MTSS may be less adapted than control subjects to tibial load. In addition, low tibial bone density has been noted in MTSS patients compared with healthy controls. The decreased tibial bone density normalizes once the athlete recovers from MTSS. Thus, tibial bones with decreased bone density may not tolerate the repeated loads experienced by athletes, resulting in MTSS, or repeated loads may themselves cause decreased bone density and lead to MTSS.
History and Physical Examination
MTSS should lead the differential diagnosis for running athletes with posteromedial tibial border pain. The pain is usually located in the middle or distal third of the tibia. Patients will often report that symptoms initially were absent at rest, began with exertion, and subsided with continued exercise. As the condition worsens, symptoms may not resolve during exercise. Pain after exertion can also manifest, but in these cases it is essential to rule out TBSI.
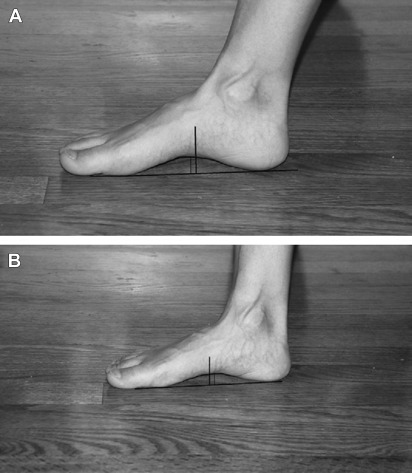
On examination, diffuse tenderness to palpation along the posteromedial distal two-thirds of the tibia is reportedly more sensitive than the pain on hopping or with percussion. Table 1 details intrinsic risk factors found to be associated with MTSS. Foot pronation can be assessed using the navicular drop test ( Fig. 1 ).
| No. of MTSS | No. of Controls | MD [95% CI] | I 2 (%) | Overall Effect | |
|---|---|---|---|---|---|
| Significant Difference | |||||
| BMI | 187 | 264 | 0.79 [0.4, 1.2] | 0.00 | P <.001 |
| Navicular drop | 198 | 366 | 1.2 [0.5, 1.8] | 40.19 | P <.001 |
| Ankle PF ROM | 71 | 166 | 5.9 [3.6, 9.2] | 0.00 | P <.001 |
| HIP ER | 117 | 162 | 3.9 [1.8, 6.1] | 0.00 | P <.001 |
| No Significant Difference | |||||
| Ankle DF ROM | 173 | 308 | −0.01 [−0.96, 0.93] | 17.89 | P =.98 |
| Ankle eversion ROM | 108 | 173 | 1.17 [−0.02, 2.36] | 31.58 | P =.06 |
| Ankle inversion ROM | 89 | 160 | 0.98 [−3.11, 5.07] | 71.58 | P =.64 |
| Q-angle | 132 | 214 | −0.22 [−0.95, 0.50] | 5.23 | P =.54 |
| Hip IR | 117 | 162 | 0.18 [−5.37, 5.73] | 83.74 | P =.95 |
Clinicians should be aware of other causes of ELP that can mimic the presentation of MTSS, the most noteworthy being TBSI and CECS. Differentiating between these 3 conditions can most often be done by obtaining a detailed history and performing a thorough physical examination. In cases where uncertainty exits, diagnostic imaging and compartment pressure testing can help clarify the underlying cause.
Diagnostic Evaluation
Imaging is not indicated for cases of uncomplicated MTSS. However, when diagnostic uncertainty exists, imaging may be warranted to confirm the diagnosis of MTSS and exclude TBSI. The use of radiography in the diagnosis of MTSS is limited, but may help rule out other causes of ELP ( Table 2 ). The high false-positive rate of bone scintigraphy and its poor ability to differentiate between MTSS and TBSI may limit its utility in the assessment of MTSS. MRI has been increasingly used over bone scintigraphy in the assessment of ELP ( Fig. 2 ). Not only has MRI been shown to accurately differentiate between MTSS and TBSI, it may also be used to guide treatment. MRI also provides information regarding other potential causes of ELP, particularly when used after exertion. The role of computed tomography (CT) in the assessment of MTSS is unclear at present.
| Grade | Clinical Diagnosis | Radiography | Bone Scan | MRI | CT Scan | Rehabilitation |
|---|---|---|---|---|---|---|
| 1 | Medial tibial stress syndrome | Gray cortex sign; margin is indistinct, density lower | Linear increased activity in cortical region | Mild to moderate periosteal edema on T2-weighted images; marrow: normal on T1- and T2-weighted images | Soft-tissue mass adjacent to periosteal surface | 2–3 wk nonimpact activity |
| 2 | Acute periosteal reaction, density differs from rest of cortex showing incomplete mineralization | Small focal region of increased activity | Grade 1+ marrow edema on T2-weighted images | Increased attenuation of yellow marrow | 4–6 wk nonimpact activity | |
| 3 | Tibial bone stress injury | Lucent areas in cortex, ill-defined foci at site of pain | Larger focal lesion with highly increased activity in the cortical region | Grade 2 + marrow edema on T1-weighted images | Increased hypattenuation (osteopenia), intracortical hypoattenuation (resorption activity), and subtle intracortical linear hypoattenuation (striation) | 6–9 wk nonimpact activity |
| 4 | Fracture line present | Very large focal region of highly increased activity | Grade 3 + fracture line clearly visible | Hypoattenuating line | 6 wk of cast, followed by 6 wk of nonimpact activity |

Management
Initial treatment includes activity modification to avoid aggravating activities, and correction of modifiable risk factors (see Table 1 ). If the navicular drop test is positive, specific measures addressing overpronation (custom foot orthosis, shoe modifications, motion control footwear, therapeutic adhesive taping, or gait retraining) should be pursued. The duration of rest from aggravating activity can be guided by MRI using the imaging criteria of Fredericson and colleagues (see Table 2 ). If an MRI has not been completed, the patient should rest until pain-free during normal daily activities and nontender over the area; this typically requires 2 to 6 weeks of rest, but can take longer if the condition is more severe.
The patient should progress from restricted to unrestricted activity in a systematic, gradual fashion. For example, the patient initially may exercise in a non–weight-bearing environment (eg, pool running) as long as he or she is pain-free. As their symptoms improve, patients may be transitioned to bicycling using a low gear and high cadence in a seated position. The next step in the progression can include higher-resistance seated or standing bicycling, or exercise on an elliptical machine. When they have no pain with these activities or with palpation over the previously tender area, patients can be transitioned to running. Running should begin on a treadmill so patients have absolute control over the pace, duration, and incline/decline of their run. If an antigravity treadmill is available, it can be used before the use of a conventional treadmill. However, clinicians should be aware that no studies have examined its use in the rehabilitation of MTSS, and a recent study noted a significant difference between the reported and measured body weight supported by the antigravity treadmill. Duration should be increased before intensity, but should not be increased greater than 10% per week. Eventually patients can transition to running outside, preferably on a track rather than concrete or asphalt, and high-intensity running workouts (eg, intervals) and plyometrics can be incorporated into their training regimen.
Extracorporeal shock-wave therapy (ESWT), when used with a graded running program, has been found to significantly decrease the time to full recovery compared with controls. In recalcitrant cases where general conservative measures and those aimed at addressing patients’ intrinsic risk factors (see Table 1 ) have failed, surgery can be considered.
Tibial bone stress injury
TBSI can involve the cortical (compact) bone located in the diaphysis of long bones, or cancellous bone (trabecular bone) located in the metaphysis and epiphysis of long bones. The term stress fracture has been supplanted by stress injury to acknowledge that TBSI can occur without a true fracture on imaging. Although the incidence of TBSI in runners has not been elucidated, in a large cohort of bone stress injuries in athletes the prevalence of TBSI was 49.1%.
Two distinct types of diaphyseal TBSI have been described. One affects the anterior tibial cortex while the other affects the posterior tibial cortex. Anterior TBSI is proposed to occur secondary to tension strain, whereas posterior TBSI is due to compressive strain ( Fig. 3 ). Both can occur proximally or distally. The exact pathophysiology of TBSI has not been elucidated, but it has been proposed that microdamage occurs when the tibia is exposed to repetitive strain, resulting in a bone remodeling response (osteoclastic activity). During this process whereby resorption precedes deposition (osteoblastic activity), the tibia is thought to be weakened, leading to a tibial stress reaction or fracture. The overarching causation for TBSI can be dichotomized into fatigue and insufficiency types. Fatigue stress injuries are related to overuse injuries in athletes with a normal bone mineral density. Two studies suggested that decreased muscle mass and decreased tibial bone cross-sectional area may be predisposing factors. Insufficiency types are related to a decreased bone mineral density, and may be more relevant in female athletes. When eliciting details on history and during physical examination, the clinician can categorize suspected intrinsic and extrinsic risk factors under these 2 groupings.

History and Physical Examination
Athletes will characteristically present with localized pain of insidious onset, which worsens with weight-bearing activities. The hallmark sign is pain after exertion, which progresses to pain during exertion. This aspect assists in differentiating TBSI from MTSS. In severe cases, pain can occur at night. While obtaining the history, recent changes in frequency, intensity, timing, and type of exercise training should be noted, as recent increases in these variables may lead to the development of TBSI. In female athletes, risk factors for the female athlete triad should also be ascertained, and can be screened for using a 12-item screening tool. The duration of running-shoe use should be noted, as it has been suggested that shoes older than 6 months can predispose a runner to TBSI.
On examination, the region of discomfort is often tender to palpation and can be swollen. In one study, a 128-Hz tuning fork was used to elicit pain by placing it on the anterior tibia (75% sensitive and 67% specific). This study used bone scintigraphy within 30 days of the tuning fork test as the gold standard, which may have weakened the strength of its findings. Limb alignment, muscle tone, and straight-leg raise range of motion should be assessed, as these may be risk factors for the development of TBSI. Caution should be exercised when excluding the diagnosis of TBSI based on physical examination findings alone if the history is suggestive of TBSI. When TBSI is included in the differential diagnosis of ELP, further imaging is essential to make this diagnosis and classify the injury.
Diagnostic Evaluation
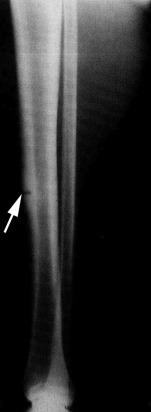
Beck and colleagues correlated radiography, bone scintigraphy, MRI, and CT in the assessment of TBSI (grade III–IV) using criteria from 4 previously published articles (see Table 2 ). Although radiography is often the first line of imaging and can reveal a fracture ( Fig. 4 ), it has low sensitivity for TBSI. Bone scintigraphy, previously thought to be the reference standard for assessing TBSI, is now discouraged in the assessment of TBSI because of its low specificity and inaccuracy in grading TBSI. MRI has been proved to be superior to radiography and bone scintigraphy in the assessment of TBSI, providing more information on the extent and level of TBSI. MRI can also be used to determine management in TBSI. Although Gaeta and colleagues have published a grading system for CT in the assessment of TBSI, its clinical role is limited by its low sensitivity. Thus, MRI is indicated in athletes with suspected TBSI whose initial radiographs are negative.
Management
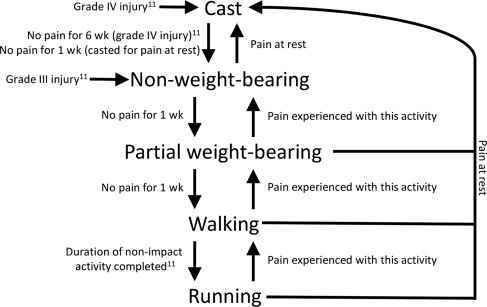
Current management strategies for TBSI are based on the Fredericson MRI criteria, whereby grade III injuries are rehabilitated with 6 to 9 weeks of nonimpact activities, and grade IV injuries are immobilized for 6 weeks followed by 6 weeks of nonimpact activity. Robertson and Wood systematically reviewed the literature investigating return to sport after diaphyseal TBSI, and found that posterior diaphyseal TBSI responded to shorter durations of nonoperative treatment (6–12 weeks), anterior TBSI required up to 6 months of nonoperative treatment and occasionally required surgery, and complete tibial fractures were treated in the same manner as an acute fracture. A Cochrane review assessing treatments for “stress fractures and stress reactions” noted a possible role for pneumatic bracing in the treatment of TBSI, leading to a quicker recovery. The rehabilitation process should be guided by pain and the Fredericson criteria ( Fig. 5 ). Surgical options can be explored with anterior TBSI in cases of delayed healing, in elite athletes, or in female athletes with a low bone mineral density and high-grade TBSI (found to have delayed recovery), as surgery may decrease recovery time. Three surgical procedures have been described (intramedullary nailing, plating, and excision and drilling), with the former 2 having a pooled return-to-play rate of 100%, and the latter a rate of 92.5%. Of the 3 surgical techniques, intramedullary nailing seems to be the procedure of choice for TBSI.
Educating athletes and training staff on the symptoms of TBSI can shorten the time from symptom onset to diagnosis, given that a delay in diagnosis can worsen the athlete’s prognosis. A Cochrane database review suggested that shock-absorbing shoe inserts may reduce the incidence of TBSI. Athletes should be screened for the female athlete triad and, when identified, treated appropriately. Vitamin D levels higher than 40 ng/mL (<50 ng/mL) may also help to prevent bone stress injuries.
Chronic exertional compartment syndrome
In athletes presenting with undiagnosed leg pain, the incidence of CECS has been reported to be between 14% and 27%. The mean age of presentation for CECS is between 26 and 28 years, with an equal distribution in males and females. Ninety percent of diabetics with of mean age 48 years with nonvascular ELP have CECS in one study. Although CECS affects the lower leg in approximately 95% of cases, it can also affect the forearm, thigh, and foot.
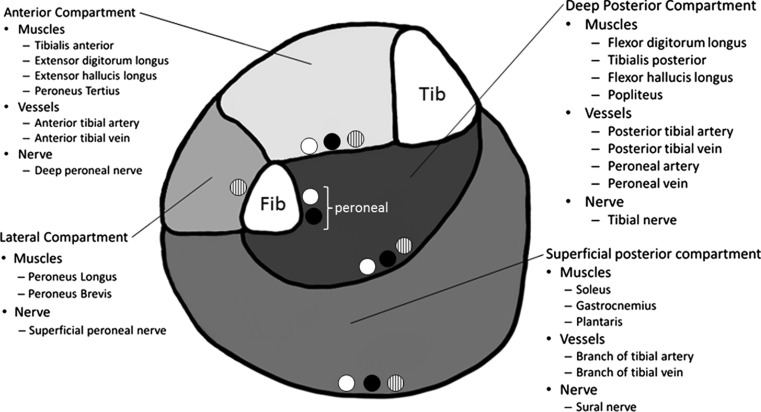
CECS can occur in any of the 4 lower leg compartments ( Fig. 6 ). The deep posterior compartment may also have a fifth compartment, containing only the tibialis posterior. CECS is reported almost equally in the anterior compartment (40%–60%) and deep posterior compartment (32%–60%); less frequently in the lateral compartment (12%–35%), which can accompany CECS of the anterior compartment; and least frequently in the superficial posterior compartment (2%–20%).
CECS was initially thought to occur secondarily to thickened compartment fascia, leading to a decrease in compliance. This theory has more recently been called into question. Other theories include reduced microcapillary capacity, venous congestion, increased interstitial volume secondary to creatine monohydrate supplementation, and stiff connective tissue in diabetic patients. Although the underlying pathology of CECS is not clearly understood, it has been shown that intracompartmental volume increases after exertion with an associated increase in compartment pressure above that seen in normal patients. The cause of the pain associated with this increase in intracompartmental pressure in CECS has also not been ascertained, but is postulated to occur secondarily to muscle or nerve deoxygenation, direct stimulation of fascial or periosteal sensory nerves, or release of local kinins.
History and Physical Examination
Athletes with CECS will often describe symptom development after a specific volume (time, distance, or intensity) of exertion involving the lower legs and feet. Symptoms can be localized to any or all of the compartments of the lower leg, and bilateral presentation is apparent in up to 82% of cases. Patients characterize the pain as a fullness or cramp-like sensation of the affected compartment, which increases in intensity with exertion. Numbness, paresthesias, and weakness in the sensorimotor distribution of the nerves in the affected compartments are possible accompanying symptoms, which may occur when the athlete attempts to continue the precipitating activity after symptom onset. Symptoms are described to cease immediately after or shortly after the precipitating activity is stopped, but over time the recovery time increases. The volume of exercise required to precipitate symptoms also decreases with time, and some patients experience decreased athletic performance the day after symptom onset. Though rare, CECS can develop into acute compartment syndrome, a limb-threatening surgical emergency. Several similarities in patient population and demographics, and presenting symptoms, were noted between CECS and functional PAES, and it has been recommended that ankle brachial index (ABI) testing with ankle provocation maneuvers be conducted in this group to rule this alternative diagnosis in or out.
The examination is completely normal at rest in CECS. Following precipitating exercise, neurologic impairments in the affected sensorimotor nerve distribution may be appreciated, and passive stretching of muscles in the involved compartment may be painful. Although fascial hernias have been described with CECS, their presence can also be seen in the asymptomatic population.
Diagnostic Evaluation
French and Price first described the use of needle manometry in CECS. Intracompartmental pressure testing continues to be a mainstay in the workup of CECS, and the preexertional and postexertional measurements published by Pedowitz and colleagues are the criteria most commonly used to diagnose CECS ( Table 3 ). Several different needle manometry devices have been described in the literature, but an international survey found the side-port needle manometer to be the most commonly used. Two systematic reviews have noted several weaknesses in needle manometry technique and the diagnostic criteria used in the assessment of CECS. Nevertheless, most clinicians continue to use this technique. A recent meta-analysis suggested that the Pedowitz criteria should be modified as follows to improve their sensitivity and specificity: preexercise greater than 14 mm Hg, 1 minute postexercise greater than 35 mm Hg, and 5 minutes postexercise greater than 23 mm Hg. No clear exercise protocol exists for compartment pressure testing, but it has been suggested that patients should exercise until maximally tolerated symptoms are produced before postexercise compartment testing.







