Fig. 15.1
Change in the number of clinical research publications in English regarding the glucose clamp technique, euglycemic-hyperinsulinemic clamp, and/or hyperglycemic clamp, from 1979 to 2014. A PubMed search was conducted using the following parameters: “glucose clamp” or “euglycemic clamp” or “hyperglycemic clamp” in any field, “subjects” or “patients” in the abstract, and English as the language
15.3 Artificial Pancreas in Japan
15.3.1 History of the Development of the Artificial Pancreas
Professor E Perry McCullagh, an endocrinologist at the Cleveland Clinic, proposed the concept of the AP in 1959, as a substitute for decreased pancreatic beta cell function and insulin secretion in response to blood glucose levels [25]. The AP is strictly the artificial endocrine pancreas (AEP) because it is unable to perform the pancreas’s exocrine functions. However, the word “artificial pancreas, AP” is adopted in this review because the STG model produced by the Nikkiso Co. Ltd. has a Japanese name “Zinkosuizou,” which corresponds to artificial pancreas and is approved by the Pharmaceuticals and Medical Devices Agency in Japan. The bedside-type AP with a closed-loop regulatory system consists of a continuous glucose monitoring system, a computer system equipped with a regulatory algorithm, and an insulin infusion system [25]. Albisser [26, 27] in 1974 in Toronto and Shichiri in 1975 in Osaka [28, 29] were the first to successfully use the AP clinically. This AP consisted of an Autoanalyzer for blood glucose determination, a minicomputer system, and a pump-drive system. After downsizing and improving the equipment, the bedside-type AP was introduced into the clinical and research fields of diabetes. Two models are available, namely, the Biostator (Miles Laboratory Inc., Elkhart, IN, USA) and the STG model (Nikkiso Co. Ltd., Tokyo, Japan). The induction of Biostator into the market started in 1983 and its production ended in 1987. The STG model was introduced to the market in 1987 and is now available in Japan. The various applications of the STG model in the clinic and in the research of diabetes and related diseases have been vigorously examined by the Osaka University and Kumamoto University study groups in Japan. Since 1988, medical insurance in Japan has approved clinical applications of the STG model on a short-term basis, including blood glucose control in diabetic coma, surgery, delivery, hypoglycemia, and the prediction of insulin requirements in special medical institutes and hospitals with specialist Japan Diabetes Association-approved diabetologists. According to the 2002 report by the Artificial Organs Registry in Japan, the STG model has been cumulatively used in 14,418 cases from 1983 to 2002. In 2002, 465 cases reportedly used the STG model, among which 29 (6.2 %) were used for glycemic control, 341 (73.3 %) for laboratory and clinical research (mainly glucose clamp [61.9 %]), and 95 (20.4 %) for animal experiments [25].
15.3.2 The STG-22 Model and the New STG-55 Model
To date, the STG-22 AP model (Fig. 15.2a) produced by Nikkiso Co. Ltd. has been used most widely. Its new model, STG-55 (Fig. 15.2b), was approved by the Pharmaceuticals and Medical Devices Agency in 2009 and was introduced into the clinic in 2010. The STG-55 models are largely improved; they are more convenient and highly precise. First, the equipment is more compact and lightweight (STG-22, 505[W] × 565[D] × 1,600[H] mm, 62 kg; STG-55, 375[W] × 425[D] × 1,630[H] mm, 36 kg). Second, a largely improved enzyme membrane has been included for continuous blood glucose measurement. Third, the complex circuits for glucose measurement were combined to one-set apron (Fig. 15.3). Fourth, an automatic priming system was introduced for the preparation of the machine. Fifth, the cost of the equipment was largely reduced. Among these improvements, the improved enzyme membrane in STG-55 has great advantages over the STG-22 model in terms of the improvement in the validity and stability of glucose measurements and the shortened calibration time in the preparation step. As stated below, the parameters of variability for the clamp using STG-55 demonstrate the same degree of validity as with STG-22 in spite of fewer subjects.
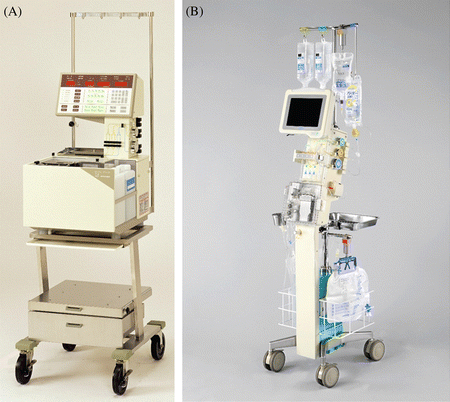
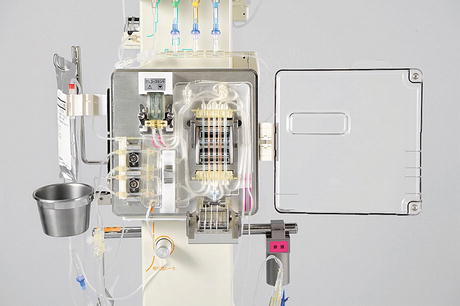

Fig. 15.2
STG-22 (a) and STG-55 (b) models of the artificial pancreas (Nikkiso Co. Ltd., Tokyo), which are widely used in Japan and have been approved by the Pharmaceuticals and Medical Devices Agency since 1987 (The photographs are provided courtesy of Nikkiso Co. Ltd.)

Fig. 15.3
One-set apron circuit for glucose measurement with the glucose and insulin infusion system in model STG-55 of the artificial pancreas (The photograph is provided courtesy of Nikkiso Co. Ltd.)
15.4 Standard Protocol for a Euglycemic-Hyperinsulinemic Clamp Using the STG Artificial Pancreas
15.4.1 Standard Protocol for a Euglycemic-Hyperinsulinemic Clamp Using an Artificial Pancreas
The euglycemic-hyperinsulinemic clamp was performed, 10–12 h after an overnight fast, according to a previously described standard protocol, using the AP models STG-22 or STG-55 [6, 8–10, 15, 16, 18]. On the morning of the study, a 20-gauge catheter was inserted into a forearm vein in a retrograde manner for constant monitoring of blood glucose levels. In the same arm, a 22-gauge catheter was inserted into the wrist vein for blood sampling. Blood sampling was performed every 30 min during the clamp study to assay the plasma insulin level. Another 22-gauge catheter was inserted into an antecubital vein of the other arm for the administration of human regular insulin (Humulin; Eli Lilly and Company, Indianapolis, IN) and a 10 or 20 % glucose solution. After baseline blood sampling, insulin was first manually infused at a priming infusion dose during the first 9 min of the clamp according to the manipulation manual for STG-22 or STG-55 and afterward automatically at a continuous rate of 1.25 mU/kg/min. The dose of constantly infused insulin corresponded to the dose in DeFronzo’s original protocol [1].
Blood glucose levels were determined every 1 min in both STG-22 and STG-55 models during the 120-min clamp study, and euglycemia (90 mg/dL, 5.0 mmol/L) was automatically maintained by infusion of different amounts of a 10 or 20 % glucose solution according to the glycemic control algorithm. The parameters for the algorithm were always set at the same constant values according to the STG-22 or STG-55 manipulation manual; in particular, setting the inputs at 1.25 mU/kg/min for insulin infusion rate was important. This is very crucial in the standard protocol as performing the glucose clamp in the same condition is required for the comparison of insulin sensitivity among subjects and/or within the same subject. If the parameters for the algorithm are set up differently for each subject or each clamp, it is difficult to compare GIR between and within subjects.
Figure 15.4 shows the schematic representative time course for monitored blood glucose, IRI, and GIR according to the aforementioned standard protocol. The real reports for a patient, a printout sheet in STG-22 and a control panel in STG-55, are shown in Fig. 15.5a, b. The various parameters for the standard protocol of a euglycemic-hyperinsulinemic clamp using the artificial pancreas, STG-22 or STG-55, are defined in Table 15.1. The M value, an index of insulin sensitivity in the original report [1], is represented as the mean of the GIRs during the last 30 min of the 120-min clamp. Finally, we use the M/I value, which is obtained by dividing the mean GIR by the steady-state plasma insulin (SSPI) levels during the last 30 min of the clamp. This is an index of insulin sensitivity as previously reported. For convenience, the M/I value is multiplied by 100.

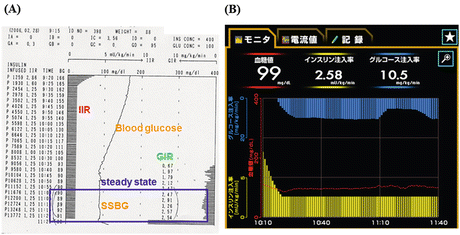

Fig. 15.4
Schematic time course of monitored steady-state blood glucose (SSBG), steady-state plasma insulin (SSPI) levels, and glucose infusion rates (GIR) according to the standard protocol for a euglycemic-hyperinsulinemic clamp using the STG model of the artificial pancreas. The bar represents mean ± SE (standard error). Priming, insulin priming infusion during the first 9 min; IIR insulin infusion rate

Fig. 15.5
Real printout sheet for a patient in the STG-22 model and control panel in the STG-55 model of the artificial pancreas. IIR insulin infusion rate, GIR glucose infusion rate, SSBG steady-state blood glucose
Table 15.1
Definitions of parameters for the standard protocol of a euglycemic-hyperinsulinemic clamp using the artificial pancreas models STG-22 or STG-55
Abbreviation | Units | Term | Definition |
|---|---|---|---|
SSBG | [mg/dL] | Steady-state blood glucose | Blood glucose level in the steady state* |
CV of SSBG | [%] | Coefficient of variance for SSBG | An index of precision of the clamp blood glucose levels |
SSPI | [μU/mL] | Steady-state* plasma insulin level | |
MCR of insulin | [/kg] | Metabolic clearance rate of insulin | Calculated as IIR × 1,000/(SSPI – (pre-IRI × post-CPR/pre-CPR)) |
GIR | [mg/kg/min] | Glucose infusion rate | Equal to M value or glucose disposal rate (mg/kg/min); mean of each glucose infusion rate in the steady state* |
SD of GIR | [mg/kg/min] | Standard deviation of GIR | An index of the stability of the GIR |
M/I value | [mg/kg/min (μU/mL)] | M value corrected by SSPI | Calculated as (GIR/SSPI) ×100; M value corrected by the plasma insulin level |
15.4.2 Precision of the Standard Protocol Using the STG Model of the Artificial Pancreas
Since 1993, we have performed the euglycemic-hyperinsulinemic clamp according to the aforementioned standard protocol using the AP model STG-22 in 394 Japanese subjects. Among these subjects, clamp studies could not be performed in only nine subjects (2.3 %) mainly because of difficulties and/or troubles with the blood sampling route in the forearm vein. Finally, GIR/M values were measured from the clamp study in 385 subjects (97.5 %). In order to examine insulin resistance in Japanese subjects with different glucose tolerances, we excluded patients with factors strongly affecting insulin resistance, morbid obesity (BMI [body mass index] greater than 35 kg/m2), chronic renal insufficiency, and type 1 diabetes. Finally, the data from 337 subjects, including 38 with normal glucose tolerance (NGT), 12 with impaired glucose tolerance (IGT), and 287 with type 2 diabetes (T2DM), were analyzed for the STG-22 model. The clinical characteristics are shown in Table 15.2. These age, BMI, and glycated hemoglobin profiles are concordant with those in Japanese patients who are admitted to hospitals for health checks/education programs.
Table 15.2
Clinical characteristics, precision of the clamp, and the insulin resistance index for the standard protocol for the euglycemic-hyperinsulinemic clamp using the models STG-22 or STG-55 artificial pancreas
STG-22 | STG-55 | |||
|---|---|---|---|---|
NGT | IGT | T2DM | T2DM | |
Characteristics of subjects | ||||
N (M/F) | 38 (25/13) | 12 (3/9) | 287 (171/116) | 14 (7/7) |
Age, years | 38.1 ± 20.2 (17–75) | 46.3 ± 15.0 | 53.3 ± 12.8 (15–74)) | 61.6 ± 13.8 (33–77) |
BMI, kg/m2 | 23.7 ± 4.2 (17.3–34.7) | 29.0 ± 3.2 | 25.2 ± 3.9 (14.6–34.9) | 26.2 ± 3.4 (20.8–32.5) |
FPG, mg/dL | 89.6 ± 8.8 | 93.0 ± 11.6 | 143.7 ± 40.7 | 121.0 ± 29.6 |
HbA1c, % | 4.87 ± 0.55 | 5.25 ± 0.43 | 8.66 ± 1.93 | 8.71 ± 1.92 |
IRI, μU/ml | 7.0 ± 6.3 | 11.1 ± 8.3 | 9.0 ± 7.1 | 10.4 ± 4.5 |
Parameters of precision of clamp | ||||
SSBGa | 92.2 ± 5.1 | 92.5 ± 1.6 | 91.0 ± 2.9 | 92.2 ± 1.7 |
CV of SSBGa | 3.56 ± 2.79 | 3.20 ± 1.85 | 2.39 ± 2.0 | 2.27 ± 2.7 |
SSPIa | 116.5 ± 45.0 | 123.6 ± 34.6 | 112.1 ± 32.4 | 114.8 ± 23.8 |
MCR of insulin | 12.49 ± 5.49 | 12.73 ± 5.39 | 12.41 ± 3.37 | 11.59 ± 2.65 |
Insulin resistance index | ||||
M or GIR | 9.43 ± 4.69 | 4.42 ± 1.9 | 4.52 ± 2.12 | 3.95 ± 1.52 |
SD of GIR | 0.85 ± 0.62 | 0.79 ± 0.47 | 0.73 ± 0.61 | 0.67 ± 0.49 |
M/I value | 9.33 ± 5.74 | 4.09 ± 2.07 | 4.56 ± 2.89 | 3.74 ± 2.31 |
For all 337 subjects, the coefficient of variance of steady-state blood glucose (SSBG) was found to be 2.49 ± 2.1 %, while in each of the NGT, IGT, or T2DM groups, it was approximately 3 %, which was within a very narrow range compared to that (around 5–10 %) for the manual program without AP (Tables 15.2 and 15.3). Because of the strict glucose clamp in the steady state, the standard deviation (SD)/standard error for each GIR in the steady state was also small indicating less variability in each subject (Table 15.3). These parameters of the clamp demonstrated the high precision of the standard protocol using STG-22. Although the precision of a euglycemic clamp has not been fully clarified in the available literature, superiority of the standard protocol using STG-22 is expected compared to the manual protocol without artificial pancreas.
Table 15.3
Insulin resistance index and parameters of precision in a euglycemic-hyperinsulinemic clamp using STG-22 according to the standard protocol in 337 Japanese subjects
Mean | SD/SE | Median | IQR | Range | |
|---|---|---|---|---|---|
SSBG | 91.2 | 3.2/0.2 | 91.3 | 2.9 | 76.0–116.8 |
CV of SSBG | 2.49 | 2.06/0.13 | 1.83 | 1.82 | 0.45–16.22 |
SSPI | 113.0 | 34.6/1.9 | 105.1 | 37.5 | 47.0–245.0 |
MCR of insulin | 12.4 | 3.6/0.2 | 12.2 | 4.3 | 5.2–27.5 |
GIR (=M) | 5.07 | 2.97/0.16 < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




