, James B. Galloway2 and David L. Scott2
(1)
Molecular and Cellular Biology of Inflammation, King’s College London, London, UK
(2)
Rheumatology, King’s College Hospital, London, UK
Abstract
RA and the seronegative spondyloarthropathies are relatively common disorders with a prevalence of approximately 1 % in European and North American populations. They are complex diseases that are considered to result from environmental exposures in genetically predisposed individuals. The main genetic risk factors are in the HLA region, with HLA-DRB1 and HLA-B27 alleles being the dominant risk factors for RA and AS, respectively. Environmental factors play an important role in RA development, particularly exposure to cigarette smoke. A broad range of immune system components are involved in the precipitation and perpetuation of the inflammatory arthropathies, particularly cytokines such as tumour necrosis factor-α. This chapter will provide an overview of the epidemiology of the inflammatory arthropathies, their underlying genetic and environmental risk factors, alongside the immunopathological changes that characterise them.
Keywords
PrevalenceComplex DiseaseHLA RiskCigarette SmokingCytokinesEpidemiology of Rheumatoid Arthritis
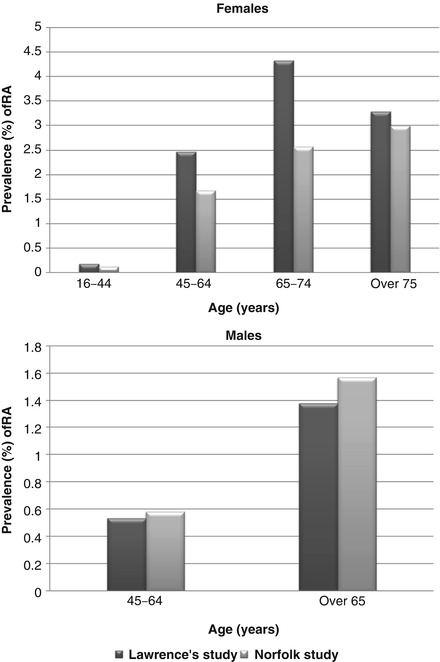
Although RA is an international problem, there is a wide geographical variation in its prevalence. It is relatively common in European and North American populations, with a widely quoted prevalence (the proportion of individuals with the disease at any given time) of 1 % in the UK. By contrast the disease is much rarer in developing countries such as Nigeria and Pakistan where its prevalence is estimated at less than 0.5 % [1]. These variations probably reflect differences in genetic risks and environmental exposures. There is some evidence that the prevalence of RA is changing. A seminal study by Lawrence in 1961 found a prevalence of RA in two Northern UK areas of 1 % [2]. A more recent study in 2002 of the Norfolk population estimated the adult prevalence of RA to be 0.81 % [3]. Comparing the prevalence of RA in the Lawrence and Norfolk studies stratified by age group and gender suggests the fall in prevalence is confined to women (Fig. 2.1).
The age distribution of RA is unimodal with a peak incidence between the fourth and sixth decade. Compared to men, women are two to three times more likely to develop RA. As RA is a chronic disease the incidence of new cases is relatively rare.
Epidemiology of Seronegative Arthritis
There is a strong association between seronegative arthritis and HLA-B27. The presence of this genotype and male sex are dominant factors in the epidemiology of seronegative arthritis. The prevalence of spondyloarthropathies in European and North American white populations may be as high as 1.5 %, though most experts consider it is below 1 %. There is an excess of males in almost all subsets of spondyloarthropathy. Geographical variation in the prevalence of spondyloarthropathies reflects variations in the number of people who are HLA-B27 positive. AS and undifferentiated spondyloarthropathy are the most frequent spondyloarthropathy subtypes. Individuals with inflammatory back pain who are HLA-B27 positive have a 50 % likelihood of having sacroiliitis.
Aetiology of Inflammatory Arthritis
The inflammatory arthropathies are considered to be complex disorders that occur when genetically predisposed individuals are exposed to environmental factors. These gene-environment risk factors interact causing changes in the immune system and a subsequent inflammatory arthritis.
Rheumatoid Arthritis
Genetic and environmental risk factors are best defined for RA, particularly the subtype in which autoantibodies − RF or antibodies to citrullinated protein antigens (ACPA) – are present, termed seropositive RA. Most genetic risk for seropositive RA is derived from the HLA region, specifically a group of alleles called HLA-DRB1. These encode the HLA class II DRβ-chain, which plays a vital role in the presentation of antigens to T cells. HLA-DRB1 alleles encoding a group of amino acid sequences (QRRAA, RRRAA and QKRAA) spanning positions 70–74 of the HLA-DRβ1 molecule particularly increase the risk of seropositive RA [4]. These alleles are known as the shared epitope (SE) alleles. A recent large analysis combining genetic studies of patients with and without RA has identified 100 genetic markers that predispose to RA development that are external to the HLA region [5].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree