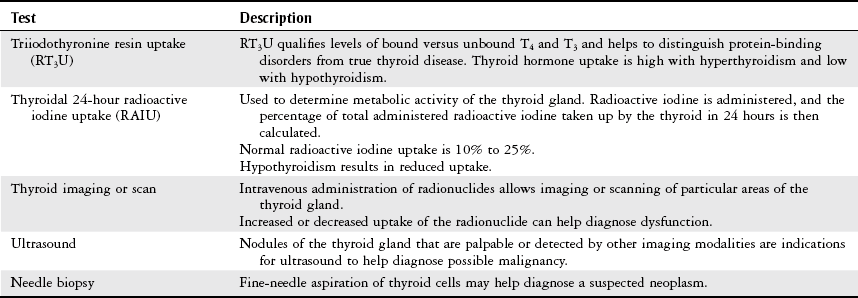
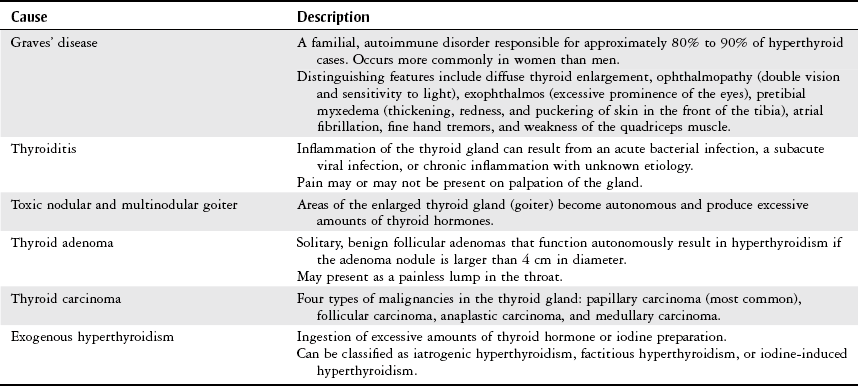
Chapter 10 The objectives of this chapter are the following: 1 Provide an understanding of normal functions of the endocrine system, including the thyroid, pituitary, adrenal, and parathyroid glands, as well as the pancreas 2 Describe the clinical evaluation of these endocrine organs 3 Describe the health conditions associated with endocrine system dysfunction and subsequent medical management 4 Provide physical therapy guidelines for working with patients who have endocrine system dysfunction • Primary Prevention/Risk Reduction for Skeletal Demineralization: 4A • Impaired Aerobic Capacity/Endurance Associated with Deconditioning: 6B • Primary Prevention/Risk Reduction for Integumentary Disorders: 7A Please refer to Appendix A for a complete list of the preferred practice patterns, as individual patient conditions are highly variable and other practice patterns may be applicable. The endocrine system consists of endocrine glands, which secrete hormones into the bloodstream, and target cells for those hormones. Target cells are the principal sites of action for the endocrine glands. Figure 10-1 displays the location of the primary endocrine glands. FIGURE 10-1 Schematic representation of the primary endocrine glands in women and men. (Courtesy Marybeth Cuaycong.) The endocrine system has direct effects on cellular function and metabolism throughout the entire body, with symptoms of endocrine dysfunction, metabolic dysfunction, or both often mimicking those of muscle weakness.1 Therefore it is important for the physical therapist to carefully distinguish the source (endocrine versus musculoskeletal) of these symptoms to optimally care for the patient. For example, complaints of weakness and muscle cramps can both result from hypothyroidism or inappropriate exercise intensity. If the therapist is aware of the patient’s current endocrine system status, then inquiring about a recent medication adjustment may be more appropriate than adjusting the patient’s exercise parameters. As a group, the prevalence of endocrine and metabolic disorders is approximately 5% of the U.S. population.2 An estimated deficit in endocrinologists to meet the demands of the population is projected through the year 2020.2 According to the Centers for Disease Control and Prevention (CDC), approximately 1.7% of the diagnoses of patients admitted to the emergency room were classified as endocrine disorders,3 4.9% of ambulatory care visits are a result of endocrine or metabolic disorders,4 and 5.3% of hospital discharges had endocrine or metabolic disorders listed as a primary diagnosis.5 Measurement of endocrine function can be performed by examining (1) the endocrine gland itself, using imaging techniques, or (2) levels of hormones or hormone-related substances in the bloodstream or urine. When reviewing the medical record, it is important for the physical therapist to know that high or low levels of endocrine substances can indicate endocrine dysfunction. A common method for assessing levels of hormone is radioimmunoassay (RIA).6 RIA is an immunologic technique for comparing levels of radiolabeled hormone with unlabeled hormone, which compete for binding sites on a given antibody. Another method of evaluation, referred to as provocative testing, can be classified into suppression or stimulation tests. Stimulation tests are used for testing endocrine hypofunction; suppression tests are useful for evaluating endocrine hyperfunction.7 The most commonly used endocrine tests are discussed in this chapter. Clinicians should refer to their particular institution’s laboratory values (generally located in the lab result section of the clinical record) for normal ranges of hormone or hormone-related substances referenced in their setting. The thyroid gland secretes three hormones: thyroxine (T4), triiodothyronine (T3), and calcitonin, with T4 and T3 commonly referred to as the thyroid hormones (Table 10-1). Thyroxine and triiodothyronine require the presence of adequate amounts of iodine to be properly synthesized. Therefore dietary deficiencies of iodine can hinder thyroid hormone production. The production and secretion of thyroid hormones are regulated by thyrotropin (also called thyroid-stimulating hormone [TSH]), which is secreted from the anterior pituitary gland. TSH levels are directly influenced by T4 levels through a negative feedback loop. Thyrotropin is further regulated by thyrotropin-releasing hormone, which is secreted from the hypothalamus.8–10 TABLE 10-1 Target Sites and Actions of Thyroid Gland Hormones Data from Brashers VL, Jones RE: Mechanisms of hormonal regulation. In McCance KL, Huether SE, Brashers VL et al, editors: Pathophysiology: the biologic basis for disease in adults and children, ed 6, St Louis, 2010, Mosby, pp 697-726; Hall S: Prescribing in thyroid disease, Nurse Prescribing 8(8):382-387, 2010. Thyroid hormones T4 and T3 circulate throughout the bloodstream bound to proteins or unbound, in which case they are metabolically active by themselves. Thyroxine-binding globulin (TBG) is one of the major thyroid transport proteins.9 Serum levels of T4 and T3 are usually measured by RIA. Table 10-2 describes the tests used to measure thyroid hormones, and Table 10-3 summarizes other tests used to measure thyroid function. TABLE 10-2 Data from Sacher RA, McPherson RA, Campos JM, editors: Widman’s clinical interpretation of laboratory tests, ed 11, Philadelphia, 2000, FA Davis, pp 741-823; Cohee L: Endocrinology. In Johns Hopkins Hospital, Arcara K, Tschudy M, editors: The Harriet Lane handbook, ed 19, St Louis, 2011, Mosby. TABLE 10-3 Data from Sacher RA, McPherson RA, Campos JM, editors: Widman’s clinical interpretation of laboratory tests, ed 11, Philadelphia, 2000, FA Davis, pp 786-793; Bastin S, Bolland MJ, Croxson MS: Role of ultrasound in the assessment of nodular thyroid disease, J Med Imaging Radiat Oncol 53;177-187, 2009; McDermott M: Endocrine secrets, ed 5, St Louis, 2009, Mosby, pp 279-282. Hyperthyroidism, or thyrotoxicosis, is primarily characterized by excessive sympathomimetic and catabolic activity resulting from overexposure of tissues to thyroid hormones. In addition, it has been reported that hyperthyroidism results in both an increased sympathetic activity with concurrent decreased vagal (parasympathetic) tone.11 Spectral analysis of heart rate variability has been shown to detect these changes and is helpful in determining the severity of hyperthyroidism.11 Heart rate variability is discussed further in Chapter 3. Patients may also present with subclinical hyperthyroidism, which may or may not lead to overt hyperthyroidism. Subclinical hyperthyroidism is defined by low TSH levels and normal T3 and T4 levels.12 The most common causes of hyperthyroidism are outlined in Table 10-4. TABLE 10-4 Common Causes of Hyperthyroidism Data from Woolf N, editor: Pathology, basic and systemic, London, 1998, Saunders, pp 863-873; Mitrou P, Raptis SA, Dimitriadis G: Thyroid disease in older people, Maturitas 70:5-9, 2011. Signs and symptoms of hyperthyroidism include the following8,10,13: • Nervousness, irritation, emotional lability, tremors, insomnia • Fatigue, weakness, increased reflexes • Palpitations, atrial fibrillation, tachycardia • Moist and warm skin, or smooth and velvety skin • Increased perspiration, heat intolerance • Diarrhea, thirst, weight loss despite increased appetite • Lid lag, retraction, or both • Finger nails that grow away from the nail bed, thinning or loss of hair • Thyroid bruit, presence of goiter • Patients may also present with subclinical hyperthyroidism Management of hyperthyroidism primarily includes pharmacologic therapy, which is summarized in Chapter 19, Table 19-42. Surgical management is indicated for patients with large goiters, large “hot” nodules or where other options have been ruled out.14 Surgical options include six different procedures that remove portions or all of the thyroid gland.15 Hypothyroidism is the insufficient exposure of peripheral tissues to thyroid hormones. It affects growth and development, as well as many cellular processes. Primary hypothyroidism is caused by decreased thyroid hormone production by the thyroid gland and accounts for the majority of thyroid disease. Secondary hypothyroidism is caused by either pituitary or hypothalamic disease resulting in reduced TSH levels.13 The following are the causes of primary and secondary hypothyroidism13,14: • Congenital maldevelopment, hypoplasia, or aplasia of the thyroid gland • Hashimoto’s thyroiditis (autoimmune inflammation) • Hypopituitarism or hypothalamic disease • Thyroid ablation from surgery, radiation of cervical neoplasms, or radioiodine therapy for hyperthyroidism General signs and symptoms of hypothyroidism vary according to the degree of thyroid deficiency. Signs and symptoms include the following8,10,13: • Lethargy, somnolence, and reduced cognitive function • Constipation and ileus (decreased motility) • Rough, scaly, dry, and cool skin, decreased perspiration, yellowish complexion • Delayed deep tendon reflexes • Weakness, muscle cramps, and aching and stiffness • Slow speech, decreased hearing • Nonpitting edema of eyelids, hands, and feet • Bradycardia with elevated systolic and diastolic blood pressure • Cardiac failure, pericardial effusion Additional laboratory findings that are associated with hypothyroidism include the following16: • Low glucose and serum sodium levels • Elevated levels of cholesterol, creatinine phosphokinase (CK-MM), serum myoglobin, lactate dehydrogenase, liver enzymes, homocysteine, and prolactin Management of hypothyroidism typically includes lifelong thyroid hormone replacement, primarily consisting of generic and brand-name variations of levothyroxine.10,13 A complete list of medications is provided in Chapter 19, Table 19-42. Hormones secreted by the pituitary gland are responsible for a variety of functions that are summarized in Table 10-5. Secretions of hormones from the pituitary gland are closely regulated by the hypothalamus and by negative feedback from the hormones that are secreted from the pituitary gland.8 TABLE 10-5 Target Sites and Actions of Pituitary Gland Hormones *Actually produced in the hypothalamus but stored in the pituitary gland. From Hall JE: Pituitary hormones and their control by the hypothalamus. In Guyton and Hall textbook of medical physiology, ed 12, St Louis, 2010, Saunders Elsevier, pp 895-906. Individual pituitary hormone levels can be measured (1) by random blood samples; (2) by blood samples before and after the administration of specific releasing substances, such as serum TSH, during a thyrotropin-releasing hormone test (see Table 10-2); or (3) by blood samples before and after the administration of specific stimuli acting directly on the pituitary or via the hypothalamus, such as serum growth hormone (GH), serum cortisol, and plasma adrenocorticotropic hormone (ACTH). Table 10-6 describes common tests of pituitary function. TABLE 10-6 Data from Malarkey LM, McMorrow ME, editors: Nurse’s manual of laboratory tests and diagnostic procedures, Philadelphia, 2000, Saunders, pp 580-584, 552-555, 613-614, 616-619; and Wilson G, Mooradian A, Alexandraki I, Samrai G: Endocrinology. In Rakel RE, Rakel DP, editors: Textbook of family medicine, ed 8, Philadelphia, 2011, Elsevier Saunders, pp 756-784. Pituitary function can also be evaluated by (1) thyroid function tests, which are an indirect assessment of TSH secretion from the pituitary, and (2) plain x-rays or computed tomography with contrast to highlight a pituitary tumor.17 Dysfunction of the pituitary-hypothalamic system generally results from hypersecretion or hyposecretion of tropic hormones. Hypersecretion of pituitary hormones (hyperpituitarism) is most commonly due to adenoma in the anterior lobe (benign tumors).18 Hyposecretion of pituitary hormones (pituitary insufficiency) can result from pituitary disease, diseases affecting the hypothalamus or surrounding structures, or disturbance of blood flow around the hypothalamus and pituitary.19,20 Excessive GH secretion is referred to as acromegaly in adults or gigantism in children. Excessive GH secretion has been linked primarily to anterior pituitary adenomas and not necessarily to excessive hypothalamic stimulation of the pituitary.20 Clinical manifestations for children with gigantism are characterized by disproportionately long limbs.18 Signs and symptoms of adults with acromegaly include the following20,21: • Enlargement of hands and feet, coarse facial features with furrowed brows • Oligomenorrhea or amenorrhea in women • Paresthesia of hands, carpal tunnel syndrome Management of acromegaly may include the following: transsphenoidal surgical resection for microadenoma (treatment of choice in both Europe and the United States) and neurosurgery for macroadenomas.22 Medical therapy consists of GH suppression with somatostatin receptor ligands (SRLs), dopamine agonists (DAs), and GH receptor antagonists (GHRA). Radiation therapy is considered third-line treatment.23 An increase in ACTH production by the pituitary gland results in increased levels of serum cortisol, which is a glucocorticoid secreted by the adrenal glands. Glucocorticoids are involved with carbohydrate, protein, and fat metabolism; therefore excess cortisol levels affect these cellular processes. Cushing’s syndrome results from glucocorticoid excess (hypercortisolism). Cushing’s disease, however, is specific to ACTH-producing microadenomas in the pituitary gland.24 Pituitary hypersecretion of ACTH occurs in approximately 70% of patients with Cushing’s syndrome. The hypersecretion of ACTH may originate either from a tumor in the pituitary gland or from ectopic neuroendocrine tumors elsewhere in the body.8,25 Signs and symptoms of Cushing’s syndrome include the following8,17,20,25: • Truncal obesity with thin extremities (loss of type II muscle fibers)18 • Redness and rounding of the face (moon face) • Easy bruising, thinning of the skin, and presence of striae and darker pigmentation • Hirsutism, oligomenorrhea, or amenorrhea • Osteoporosis (radiographically confirmed) • Glucose intolerance, glycosuria, and polydipsia resulting in diabetes mellitus Diagnosis of Cushing’s syndrome is established by increased levels of urinary cortisol in a 24-hour period.18 Management of Cushing’s syndrome may include the following: surgical resection of pituitary lesion, radiation, or gamma-knife surgery.25 Medical management may consist of steroidogenic inhibitors (ketoconazole) or neuromodulators of ACTH release with somatostatin analogs and dopamine agonists.25 The syndrome of inappropriate ADH (SIADH) secretion is a condition of fluid and electrolyte imbalance resulting in hyponatremia (reduced sodium levels) from excessive water reabsorption. In this condition, ADH is secreted from the posterior pituitary gland when it should be inhibited. Hyponatremia is fairly common in hospitalized patients and can result in significant morbidity and mortality.26 Numerous etiologies of SIADH exist, with the most frequent cause being small cell or oat cell carcinomas of the lung. Other etiologies include the following27,28: • Bacterial pneumonias, chronic obstructive pulmonary disease, tuberculosis, lung abscesses • Malignancies of the pancreas, duodenum, colon, lymphoid tissue, and thymus • Medication side effects from antipsychotics, sedative-hypnotics, antidepressants, diuretics, antihypertensives, analgesics, cardiac drugs, nonsteroidal antiinflammatory drugs, and antibiotics29 Mild SIADH is usually asymptomatic. More severe cases, however, can result in fluid and electrolyte imbalances, resulting in interstitial edema from a lack of serum sodium. Many systems will be affected by this edema, with the nervous system being most severely involved. Manifestations may include the following: headaches, nausea, confusion, gait disturbances, falls, and cerebral edema that leads to seizures and coma (in severe cases).18,26,27 Management of SIADH may include the following: treatment of the underlying cause, fluid restriction, or administration of select agents such as demeclocycline, lithium, and urea. Intravenous administration of sodium chloride (saline) solution, or administration of diuretics (furosemide) may also be used as initial therapies but not for long-term management.26,27,29 The developing use of vaptans or vasopressin-2 receptor antagonists for mild to moderate cases of SIADH has resulted in good outcomes.26,30 Decreased secretion of pituitary hormones can result from either pituitary or hypothalamic dysfunction. Complete anterior pituitary hormone deficiency is referred to as panhypopituitarism.31 Most cases of pituitary hypofunction arise from destructive processes involving the anterior pituitary, such as ischemic necrosis occurring during the late stages of pregnancy (Sheehan’s syndrome). Additional causes may include pituitary adenomas, traumatic brain injury, pituitary surgery, or radiation.31 Symptoms and physical findings depend on the extent of the disorder and the specific hormone (GH, TSH, or ACTH) and target cells involved, such as32: • Decreased libido, impotence with loss of pubic and axillary hair in men • Amenorrhea and infertility in women Management of panhypopituitarism may include the following: replacement therapy or pituitary hormones, such as thyroxine, glucocorticoids, and GH for children; desmopressin for DI; androgen therapy for men; or estrogen therapy for women younger than 50 years of age. Management of other clinical sequelae of hypopituitarism is specific to the involved areas.17,33 DI involves the excretion of a large volume (i.e., greater than 50 ml/kg per day) of dilute urine (hypotonic polyuria). DI may result from an absence of vasopressin or an inadequate response to vasopressin. Four categories of DI exist: primary polydipsia, central (hypothalamic) DI, gestational DI, and nephrogenic DI.34 Signs and symptoms of DI may be transient or permanent, and include the following28,33,35,36: • Polyuria, nocturia with resultant hypernatremia (increased sodium) • Thirst (especially for cold or iced drinks), polydipsia • Dry skin with decreased turgor • Central nervous system manifestations (e.g., irritability, mental dullness, ataxia, hyperthermia, and coma) if access to water is interrupted Management of DI may include pharmacologic treatment, such as the following: deamino-8-D-arginine vasopressin (desmopressin), chlorpropamide (Diabinase), clofibrate (Atromid-S), carbamazepine (Tegretol), and thiazide diuretics in combination with a sodium-restricted diet.37 The adrenal gland has two distinct areas, the outer cortex and the inner medulla, that differ in their function and embryologic origin.8 Table 10-7 summarizes the target sites and actions of the adrenal gland hormones. TABLE 10-7 Target Sites and Actions of Adrenal Gland Hormones *Blood levels unless otherwise specified. †Aldosterone levels vary from supine to upright positions. Data from Fuller BF: Anatomy and physiology of the endocrine system. In Hudak CM, Gallo BM, editors: Critical care nursing: a holistic approach, ed 6, Philadelphia, 1994, Lippincott, p 875; Corbett JV, editor: Laboratory tests and diagnostic procedures with nursing diagnoses, ed 5, Upper Saddle River, NJ, 2000, Prentice Hall Health, p 391; Pagana KD, Pagana TJ: Mosby’s diagnostic and laboratory test reference, ed 9, St Louis, 2009, Mosby. Evaluation of the adrenal cortical (glucocorticoids, androgens, and mineralocorticoids) and medullary (epinephrine and norepinephrine) hormones is typically performed by measuring plasma and/or urinary levels of the hormone in question. Reference values for adults for each hormone are listed in Table 10-7. The primary method for evaluating adrenal activity is by measuring plasma cortisol levels. Levels are usually drawn at 8 AM and then 4 PM to evaluate whether there is any deviation from the expected diurnal variation. Cortisol levels peak in the morning and taper during the rest of the day. Increased levels indicate Cushing’s syndrome, decreased levels suggest Addison’s disease. Normal serum levels are 5 to 23 mcg/dl (8 to 10 AM) and 3 to 13 mcg/dl (4 to 6 PM).38,39 Analysis of urine over a 24-hour period is used to determine the urinary levels of free cortisol. Normal levels are less than 100 mcg/dl for a 24-hour period.38,39 ACTH levels are usually examined concomitantly with cortisol levels, as ACTH secretion from the pituitary gland is necessary for cortisol secretion from the adrenal glands. Refer to Table 10-6 for details on ACTH measurement. Anatomic investigation of the adrenal glands may also be performed to diagnose possible adrenal dysfunction. Common methods to accomplish this are computed tomography scan (to identify adrenal tumors), radioisotope scan using selenocholesterol, ultrasound, arteriogram, adrenal venogram (allows measurements of hormone levels), and intravenous pyelogram (see the Diagnostic Tests section in Chapter 9).38 One of the criteria of the National Diabetes Data Group is to have a glucose tolerance test (GTT) result that presents with normal fasting blood glucose and a peak GTT and a 2-hour value of greater than 200 mg/dl on more than one occasion.39 To perform the test, a 75-g or 100-g glucose load is given to the subject in the morning after a 12-hour fast. Blood glucose levels are then measured at variable time periods, ranging from every half hour to every hour for the next 2 to 4 hours after the glucose administration. The subject must remain inactive and refrain from smoking throughout the duration of the test. Normally, the blood glucose levels should fall back to baseline values in a 2-hour period. Normal glucose value for a fasting blood sugar (BS) is less than approximately 110 mg/dl. After 1 hour of ingesting the glucose load, expected levels of glucose are less than 200 mg/dl and 70 to 115 mg/dl after 4 hours.39 Autoimmune dysfunction can lead to destruction of the adrenal cortex (i.e., primary adrenal insufficiency or Addison’s disease). Additionally, ACTH deficiency from the pituitary gland can lead to atrophy of the adrenal cortex (secondary adrenal insufficiency). The net result is an impaired adrenal system with decreased levels of glucocorticoids (cortisols), mineralocorticoids (aldosterone), and androgens. Given the systemic functions of these hormones, Addison’s disease can have severe consequences if left untreated. Fortunately, the incidence of Addison’s disease is rare.40 Cortisol deficiency results in decreased gluconeogenesis (glucose production), which in turn alters cellular metabolism. Decreased gluconeogenesis also results in hypoglycemia and decreased ability to respond to stress. Aldosterone deficiency causes fluid and electrolyte imbalance, primarily as a result of increased water excretion that leads to dehydration.41,42 Symptoms and physical findings common to adrenal insufficiency include the following40,43:
Endocrine System
Preferred Practice Patterns
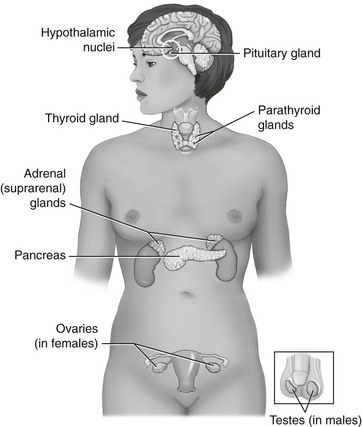
General Evaluation of Endocrine Function
Thyroid Gland
Body Structure and Function
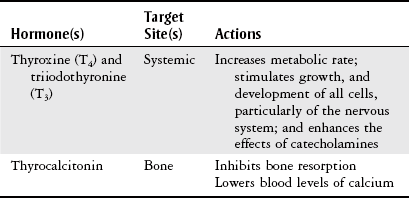
Thyroid Tests
Hormone
Test Description
Normal Value (adults)
Serum thyroxine (T4)
Radioimmunoassay (RIA) measurement.
4-12 mcg/dl
Serum triiodothyronine (T3)
RIA measurement.
40-204 ng/dl
Free thyroxine index
Direct RIA measurement or indirect calculated measurement.
0.93-1.71 ng/ml
Thyroid-stimulating hormone (TSH)
Radioisotope and chemical labeling measurement.
0.4-4.5 µU/ml
Thyrotropin-releasing hormone (TRH)
Intravenous administration of TRH to patients.
TRH augments the function of TSH in patients with hypothyroidism.
Only performed in difficult diagnostic cases.
The expected response is a rise in TSH levels.
Normal rise in men and women is 6 µU/ml above baseline TSH levels.
Normal rise in men older than 40 years is 2 µU/ml above baseline.
Hypothyroidism is indicated by increased response to TRH.
Hyperthyroidism is indicated by no response to TRH.
Thyroid Disorders
Hyperthyroidism
Hypothyroidism
Pituitary Gland
Body Structure and Function
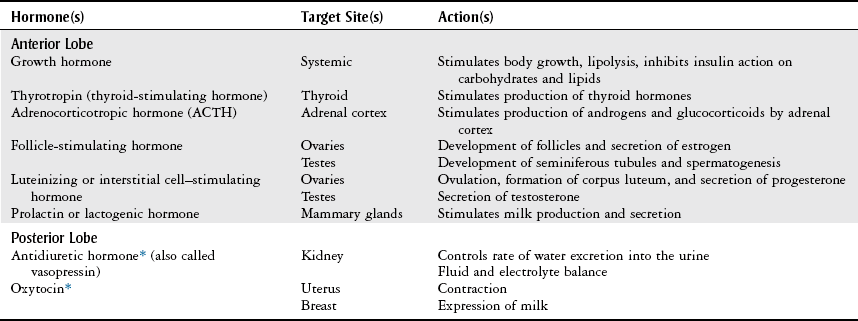
Pituitary Tests
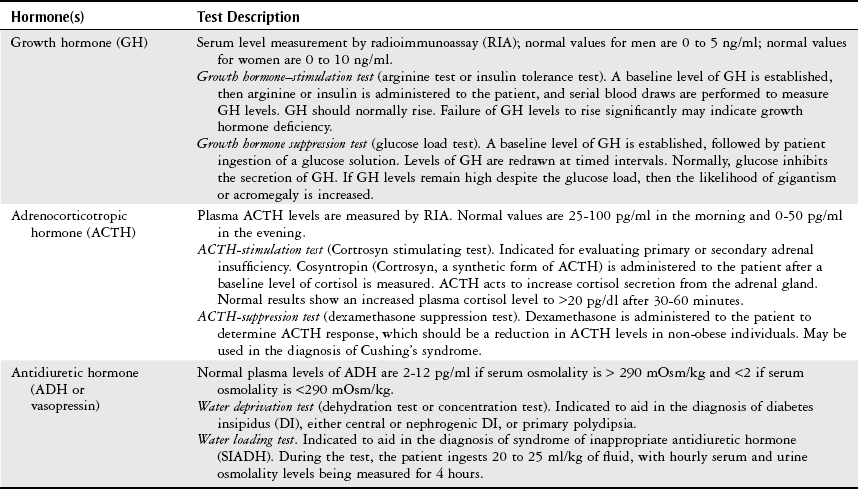
Pituitary Disorders
Hyperpituitarism
Growth Hormone Overproduction.
Adrenocorticotropic Hormone Overproduction.
Antidiuretic Hormone Overproduction.
Hypopituitarism
Diabetes Insipidus
Adrenal Gland
Body Structure and Function
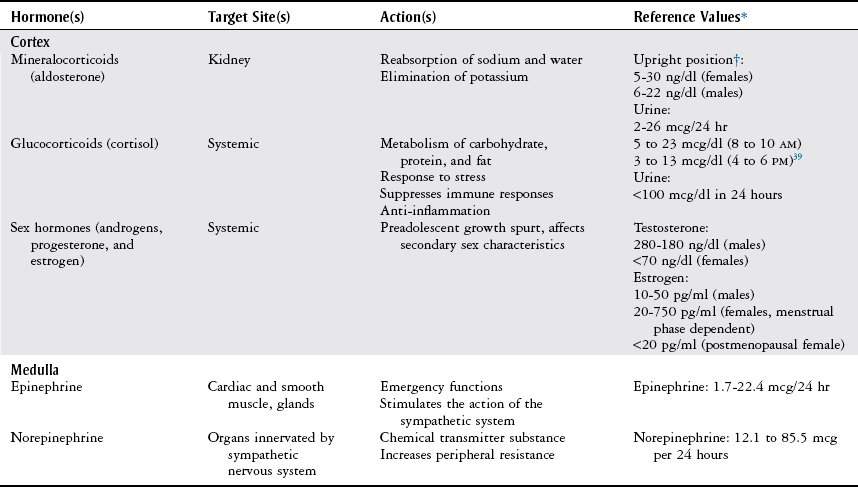
Adrenal and Metabolic Tests
Adrenal Tests
Metabolic Tests
Glucose Tolerance Test.
Adrenal Disorders
Adrenal Insufficiency
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Endocrine System
Only gold members can continue reading. Log In or Register to continue





