Chapter 14 Emerging Technologies
Marrow stimulation technique augmentation
BST-CarGel
Background
BST-CarGel (BioSyntech Canada, Laval, Quebec, Canada), a new medical device for cartilage repair, was developed to improve outcomes of bone marrow stimulation procedures while preserving the intrinsic low cost and simple arthroscopic approach. With traditional marrow stimulating techniques, clot shrinkage and detachment from lesion surfaces occur. BST-CarGel was designed to stabilize the blood clot in the cartilage lesion by dispersing a soluble and adhesive chitosan scaffold throughout autologous, fresh whole blood. Chitosan, a cationic linear polysaccharide composed predominately of polyglucosamine, is derived from the deacetylation of chitin, the structural component of crustacean shells.1,2 By dissolving chitosan in an aqueous glycerol phosphate buffer, BST-CarGel is uniquely obtained as a liquid chitosan solution having physiologic pH and osmolarity, intrinsic cytocompatibility, and biodegradability.2,3 When mixed with blood (BST-CarGel-to-blood ratio of 1:3), the viscous mixture can easily be applied to cartilage lesions that have been prepared by bone marrow stimulation (microfracture, drilling), where it permits normal clot formation while simultaneously reinforcing the clot and impeding clot retraction. Furthermore, the cationic nature of the chitosan increases the adhesivity of the mixture to cartilage lesions, ensuring longer clot residency. This maintenance of critical blood components above the marrow access holes allows activation of the tissue repair process, while chitosan itself brings an intrinsic ability to stimulate wound repair.4 Box 14–1 summarizes the primary mode of action for BST-CarGel, an approach that has been termed scaffold-guided regenerative medicine, where BST-CarGel provides in situ chondroinduction for cartilage repair.5
Nonclinical Studies
The efficacy and underlying mechanisms of action of BST-CarGel were examined in several animal studies. A skeletally mature (8–15 months) rabbit model that used drilling of surgically prepared bilateral trochlear defects elucidated BST-CarGel early reparative events and compared them with drilled controls.6,7 Box 14–2 summarizes the key findings of BST-CarGel–mediated cartilage repair.
BOX 14–2 BST-CarGel Stabilized Blood Clots
Mechanisms of Action for Cartilage Repair
Chitosan clearance via neutrophil phagocytosis after approximately 1 month
Chemotaxis of marrow stromal cells toward the lesion
Transient increase in vascularization in subchondral bone
Greater porosity, remodeling, and vascularization of subchondral bone
Induction of chondrogenic foci from repair tissue near 1 month
Increased quality of hyaline cartilage repair arising from subchondral bone
Improved integration of repair cartilage within the lesion
All comparisons were made to drilled-only contralateral controls (n = 49).11,12
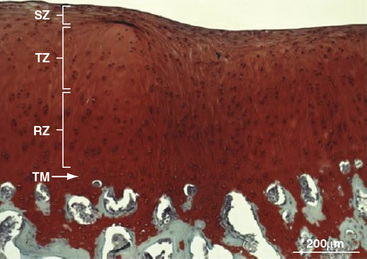
In another large study in adult (3–6 years) sheep, repair of surgically prepared 1-cm2 condylar and trochlear defects was investigated by measuring the quantity and quality of BST-CarGel repair tissue after 6 months compared with microfracture-only defects.8 Box 14–3 summarizes the efficacy of BST-CarGel treatment for cartilage repair. Figure 14–1 shows a best case repair from a sheep condylar lesion treated with BST-CarGel. After 6 months of repair, a relatively mature articular cartilage containing superficial, transitional, and radial zones with a reestablished tidemark was observed.
BOX 14–3 BST-CarGel Stabilized Blood Clots
Six-Month Efficacy in Sheep
Greater adhesion of CarGel blood clot to bone and cartilage
Increased volume of repair tissue
Improved hyaline character of repair tissue
Increased GAG and collagen content of repair tissue
Reduced incidence of subchondral cyst formation
No treatment-specific safety issues
Overall, the BST-CarGel device has been shown to statistically increase the volume and hyaline character of repair tissue compared to microfractured or microdrilled controls. The chondrogenic foci observed resemble endochondral processes and are believed to be responsible for the synthesis of cartilaginous repair tissue. Notably, the specific mechanisms underlying BST-CarGel–mediated repair (see Boxes 14–2 and 14–3) were independent of species or bone marrow stimulation technique.7
Clinical Experience
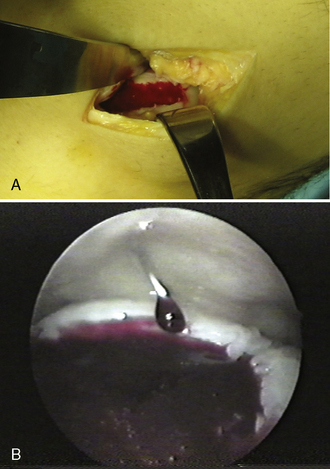
In 2003 and 2004, 33 human subjects were treated with BST-CarGel under Health Canada’s Special Access Program for medical devices (compassionate use on a case-by-case basis; not considered a clinical trial). Treated patients encompassed the spectrum of both traumatic and degenerative lesions, along with other pathologies. Lesions ranged in size from 0.5 to 12 cm2 (mean 4.3 cm2). In 16 cases, opposing tibial lesions (kissing lesions) were debrided and treated with microfracture only. One case of osteochondritis dissecans and one exposed subchondral cyst were treated; two concomitant anterior cruciate ligament replacements were performed. BST-CarGel delivery by arthroscopic (22 patients) as well as mini-open approaches (11 patients) was confirmed (Figure 14–2). Physiotherapy follow-up was standardized and required 6 weeks of non–weight-bearing exercise and early passive range of motion by physiotherapists (i.e., no continuous passive motion). Western Ontario McMaster (WOMAC)9 osteoarthritis index questionnaires were administered preoperatively and again postoperatively after 3, 6, and 12 months. WOMAC scores for pain, stiffness, and function improved substantially over preoperative baseline scores, although the absence of a control group and the wide-ranging patient demographics and lesion types prevent overinterpretation of outcomes.5
In 2005, BioSyntech initiated a multicenter randomized level 1 clinical trial in Canada and Europe comparing treatment with BST-CarGel to microfracture in the repair of grade 3 or 4 articular cartilage lesions on the femoral condyles in the knee. The study was designed to measure repair tissue structure at 12 months as the primary endpoint through quantitative magnetic resonance imaging (MRI) of repair tissue volume and quality (T2, delayed gadolinium-enhanced magnetic resonance imaging of cartilage [dGEMRIC]) and microscopic analysis of biopsies (when available). The secondary endpoint assessed clinical benefit (Visual Analog Scale [VAS], WOMAC, Medical Outcomes Study 36-Item Short-Form Health Survey [SF-36]) and safety. The trial enrolled 80 patients, and an interim histologic analysis of 22 available biopsies (13 BST-CarGel and 9 microfracture patients) using International Cartilage Repair Society Histological Scoring systems I and II10 provided statistically significant evidence that BST-CarGel improved the quality and quantity of repair tissue compared to microfracture. The International Cartilage Repair Society (ICRS) II overall score, which assimilates all the parameters listed in the grading system and generates an overall assessment of tissue repair, was significant (P < .05), as were individual parameters of cell morphology, cell viability, and superficial zone morphology on the biopsies. Macroscopic grading of the cartilage repair by the surgeon at the time of biopsy, which included the extent of lesion filling, tissue surface characteristics, and integration with surrounding tissue, also was statistically significant. Full study analysis on all 80 patients after 12-month follow-up will be conducted in the spring of 2010, followed by Canadian and European submissions for device marketing approval. A similar pivotal study will be conducted in the United States for Food and Drug Administration (FDA) approval.
Scaffolds
MaioRegen
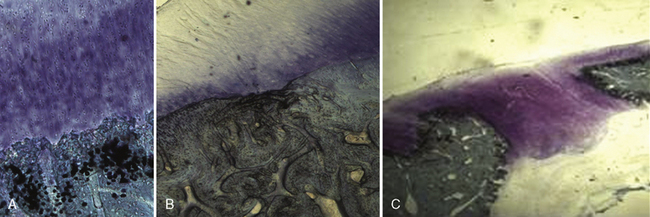
MaioRegen (Fin-Ceramica Faenza S.p.A., Faenza, Italy) is a novel composite osteochondral monolithic scaffold. It is a multilayered structure that reproduces the chemical gradient naturally found inside osteocartilaginous compartment.13,14 MaioRegen is composed of equine tendon-derived type I collagen on the upper side, which mimics the cartilaginous layer, and a blending of type I collagen and magnesium-enriched nonstoichiometric hydroxyapatite (Mg-HA) on the bottom side, which mimics the subchondral bone (Figure 14–3). Due to its high similarity with the anatomic osteocartilaginous portion to be replaced, in terms of chemical, biologic, and structural composition, the scaffold is defined as a biomimetic device, able to be recognized as self by the recipient connective tissues. Toxicologic profile, performed in compliance to EN ISO 10993–1 European regulation on class III medical devices, showed high biocompatibility and tolerability.
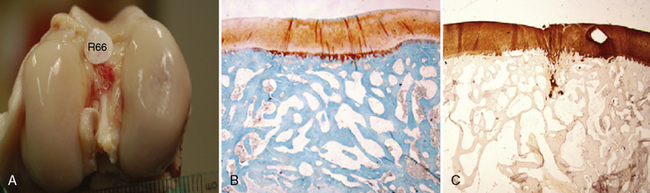
An in vivo, randomized controlled study has been performed to assess the safety and efficacy of MaioRegen in an osteochondral reconstruction sheep model.15 An osteochondral lesion was induced in the right knee, either on the medial or lateral condyle, of each animal (n = 8). Animals were then randomly assigned to three treatment groups. The objective of the study was to demonstrate a substantial equivalence (in terms of effectiveness) between the acellular approach using MaioRegen alone (group A) and chondrocytes cultured on the scaffold (cell-engineered scaffold, group B). Comparisons were made with an untreated control group (group C). No adverse events were observed in all groups, and the device was completely tolerable and fully biocompatible. At 6 months postoperative, animals were euthanized, and macroscopic and histologic investigations showed complete absorption of MaioRegen. The newly formed tissues in the treated groups were well integrated, whereas a gap on the defect was still noticeable in the control group. Applying a Fortier score (0–15),16 no statistical differences in average score values were seen between scaffold alone (2.63 ± 0.71) and cell-engineered scaffold (4.00 ± 0.53), whereas both groups exhibited statistical difference (P < .05) versus the control group (12.88 ± 0.95). Histologic evaluation showed newly formed tissue that was well organized and characterized by chondrocytes with tangential orientation in the upper part. In the two treated groups, tissues were differentiated at either the chondral or subchondral bone level, whereas fibrous tissue was evident in the control group (Figure 14–4).
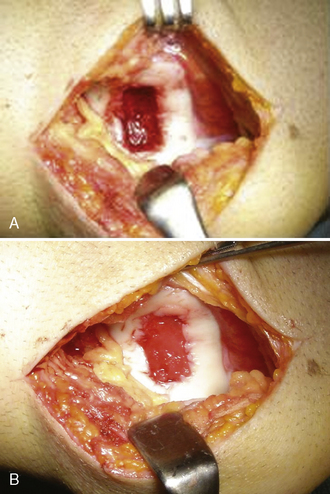
The hypothesis of a scaffold-guided tissue regeneration process is that mesenchymal and progenitor cells from subchondral bone marrow blood after surgical curettage are able to migrate inside and wholly colonize the scaffold, then differentiate along either a chondrogenic or osteogenic lineage based on the peculiar physical–chemical gradient composition found. Therefore, MaioRegen itself promoted osteoblast differentiation and bone regeneration in the deepest portions while restoring the tidemark in the intermediate portion and hyaline cartilage formation in the upper surface (chondrogenic differentiation). After the preclinical and toxicologic studies, a pilot, uncontrolled, prospective clinical trial of 30 patients has been designed and approved by an institutional review board to investigate the performance and safety of MaioRegen. Inclusion criteria for study admission were patients ranging in age from 15 to 60 years who were affected by traumatic, posttraumatic, or degenerative osteochondral defects of the knee (grade III and IV Outerbridge classification) sizing 1–9 cm2. After arthrotomy, a curettage of the defect was made, and scaffold was implanted dry by simple press fit, without fixation with suture or surgical glue (Figure 14–5). Early stability of MaioRegen assessed 30 days postoperative by MRI revealed neither migration from the implant site nor delamination. With regard to the safety outcome, patients were monitored for the onset of any adverse events. Most of the episodes reported were considered related to surgery and not to the device itself, and most occurred within the immediate postoperative period. In addition, clinical assessment by MRI performed 6 and 12 months postoperative evaluated the quality of regenerated osteochondral tissue and integration with recipient tissues applying the MRI MOCART Scoring System. Functional joint recovery, improvement of patient’s quality of life, and return to normal sport activity were further evaluated by applying ICRS evaluation package questionnaires and Kujala and Tegner scores. Results of statistical data analysis highlighted positive results for each observed variable with respect to both surgeon and patient evaluations at each follow-up visit. Modifications of the result for each examined variable, comparing 6- and 12-month postsurgery scores to the preoperative score, were statistically significant (P < .05). Upon study planning, the modification of study variables between preoperation and postoperation was identified as a success index. Because modifications in the study were reported as positive (i.e., sustained by statistical relevance), they were considered indexes of study success. Moreover, all of the parameters evaluated improved significantly after 12 months compared to 6 months, confirming good progression and enhancement of quality of life.
The preliminary results of the clinical trial at intermediate follow-up appear promising.17,18 MaioRegen may be considered a potential innovative surgical treatment of severe osteochondral lesions; however, longer follow-up would be essential to fully validate this one-step acellular approach.
Chondro-Gide
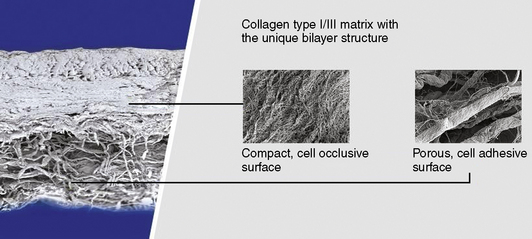
The proprietary manufacturing process of Chondro-Gide involves several steps before the unique bilayer design consisting of a compact side and a porous side is achieved (Figure 14–6). Standardized processes under clean room conditions and rigorous in-process and end controls guarantee a high-quality natural product. Chondro-Gide consists of porcine type I and III collagen, which is naturally resorbed. Collagenases, gelatinases, and proteinases are responsible for the breakdown of Chondro-Gide into oligopeptides and finally single amino acids.
Autologous Chondrocyte Implantation
Preclinical Results for Chondro-Gide in ACI
Ovine animal models and preclinical culture condition studies have validated the proof of concept that a porcine collagen membrane may be successfully used in cartilage repair with good tissue repair and no rejection response.19–24
Autologous Matrix-Induced Chondrogenesis
An initial study by Behrens et al33 showed that costs were significantly reduced by AMIC compared to ACI, and immediate cartilage repair was possible in a single procedure. Improved tissue repair to ACI has not yet been studied.
Preclinical Results for AMIC
Preclinical animal work confirms that marrow clot is contained and repair tissue develops.34,35
Clinical Results for AMIC
Numerous early reports on the femoral condyles support positive short-term clinical results.36–41
Kensey Nash Corporation Cartilage Repair Device
Implant Description
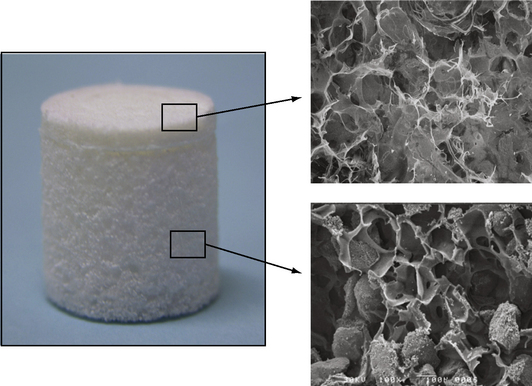
The Kensey Nash CRD is a porous biphasic scaffold comprised of type I collagen, β-tricalcium phosphate (β-TCP), and polylactic acid (PLA), three well-known biomaterials with a long history of use in orthopedics. The implant (Figure 14–7) has 100% interconnected porosity that enables movement of cells and biologic fluids throughout the entire implant. The chondral phase of the implant consists of a type I collagen formulation. The collagen provides a malleable, biocompatible scaffold for repair of cartilage tissue that can be contoured to the surrounding joint surfaces. The implant’s subchondral bone phase contains 80% β-TCP and 20% PLA by mass. The ceramic provides an osteoconductive element to the subchondral phase of the CRD implant while supplying a source of calcium and phosphorous ions necessary for natural bone mineralization.42,43 The PLA scaffold provides biomechanical support and three-dimensional structure for this region and ultimately breaks down into natural body metabolites via the Krebs cycle.44,45 As a result of Kensey Nash’s proprietary process, the ceramic granules are suspended within, not coated by, the polymer scaffold so that the ceramic is immediately available to the host upon implantation. The implant is available in a range of diameters and is loaded in an insertion tool that facilitates implant hydration and arthroscopic placement.
Preclinical Studies
Kensey Nash conducted a caprine study that evaluated the performance of the CRD in a 6-mm × 6-mm defect on the medial femoral condyle of 38 Nubian Cross goats at 6-, 12-, and 18-month time points (Figure 14–8). Histology, biomechanical testing, immunohistochemistry, MRI, gross evaluations, and radiographs were the evaluation methods used. The study concluded that, by 6 months, CRD-treated defects displayed a rapid repair that was sustained through the 12-month46 and 18-month time points. Repair tissue at each time point appeared hyaline, stained positively for type II collagen, and was biomechanically similar to healthy articular cartilage. The repair tissue also displayed excellent integration not only with the host cartilage but with the host bone as well.
Kensey Nash is also studying the performance of the CRD in healing a 10-mm × 10-mm osteochondral defect on the lateral trochlear ridge of 12 horses (Figure 14–9). The repair of CRD-treated defects will be evaluated over a 2-year study, with interim arthroscopies at 4- and 12-month time points. Interim arthroscopies are scored using a modified ICRS system for safety and efficacy. At time of sacrifice, histology, biomechanical testing, immunohistochemistry, MRI, gross evaluations, and radiographs will be used to evaluate the repair compared to a microfracture control. Four-month interim arthroscopy showed equivalency to microfracture in terms of safety scores and a significant improvement over microfracture in efficacy scoring.47
TRUFIT CB for Cartilage Repair
Properties of TRUFIT CB
The TRUFIT CB scaffold (Smith & Nephew, Andover, MA) is a cartilage repair option for small chondral or osteochondral lesions. It comes in preformed cylindrical plugs with diameters ranging from 5 to 11 mm. This synthetic resorbable scaffold has two phases to mimic the critical portions of osteochondral healing. Each phase is designed to initially approximate the physical and mechanical properties of the adjacent cartilage and bone (Figure 14–10). TRUFIT CB is made of Polygraft, a porous material composed of 85:15 poly(d,l-lactide-co-glycolide) copolymer, polyglycolide (PGA) fibers, calcium sulfate, and a trace amount of surfactant. Slivka et al48 showed that PGA reinforcement fibers improved the early structural integrity of the scaffold and provided a mechanically stable environment for cell migration and tissue repair. Calcium sulfate in the bone phase releases calcium ions, which are known to enhance osteoconductivity,49,50 whereas the top phase is malleable to allow contouring with the adjacent articular surface after implantation. At the surgery, the original chondral/osteochondral defect is cored out with TRUKOR instrumentation to create a uniform circular defect. The implant is press fit into the defect site using the preloaded delivery device. Because the TRUFIT CB scaffold is hydrophilic, fluids such as blood and marrow containing nutrients, proteins, and cells can be easily wicked up into the pores. TRUFIT CB then acts as a scaffold for microfracture to hold the blood and marrow cells from subchondral bleeding. Its 70% porosity and interconnected pores promote cell infiltration across the scaffold and provide a mechanically stable environment for tissue repair. TRUFIT CB is currently sold in Europe, Canada, and Australia but is not available in the United States and its territories.
Preclinical Study Using an Ovine Osteochondral Model
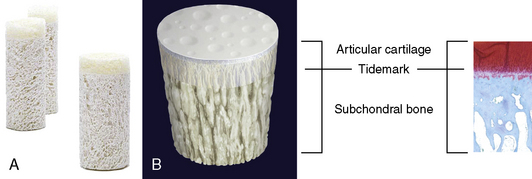
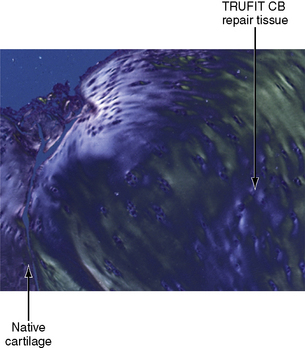
In an ovine study assessing cartilage repair by TRUFIT CB implants in osteochondral defects at 18 months, a unilateral circular (5.1 mm diameter × 5 mm) osteochondral defect was created in the medial femoral condyle of skeletally mature sheep.51 The defects were repaired by TRUFIT CB (5.3 mm diameter × 5 mm; n = 6) press fit into the site or left empty in the empty defect control group (n = 3). Outcome of cartilage repair was assessed 18 months after surgery. None of the sheep showed any lameness and had regained a full range of motion. The medial condyle of all animals in the TRUFIT CB group had no abnormalities. By 18 months, the material of TRUFIT CB had predominantly resorbed, and the defect sites were filled with repair tissue. Cartilage restoration was evident in all TRUFIT CB specimens. The repaired cartilage was well integrated with the adjacent tissue and had a similar thickness and contour (Figure 14–11). Safranin O staining revealed a highly cellular tissue in the repair by TRUFIT CB with the same intensity of proteoglycan staining as the adjacent hyaline cartilage. The organized collagen patterns of the mid and deeper levels of TRUFIT CB repair were similar to those of normal cartilage (Figure 14–12). In contrast, repair cartilage in the empty defect control was thinner than normal cartilage tissue, and the adjacent cartilage showed signs of degeneration, including proteoglycan loss (see Figure 14–11). The subchondral bone portion of the defect repaired by TRUFIT CB was replaced by bone with a statistically similar bone volume as the untreated contralateral control (66.4% and 63.0%, respectively). Areas of new bone growth with active osteoblasts, increased cellularity, and nonlamellar structure were observed in the repair, indicating that subchondral bone remodeling was ongoing and expected to continue to completely remodel to mature bone over time.
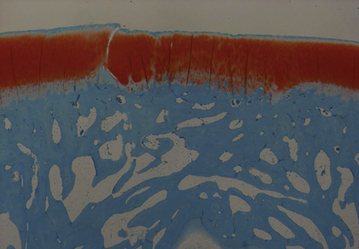
Figure 14–11 Safranin O–stained sections of TRUFIT CB–treated defect and empty osteochondral defect.
Clinical Case Series
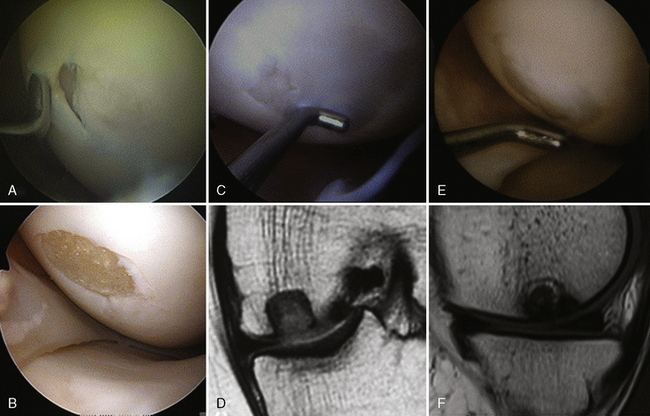
A prospective series by the Spalding group reported the outcome of 24 patients (mean age 34 years, range 19–50 years) with 12- to 36-month follow-up for treatment of chondral or osteochondral lesions in the knee with TRUFIT CB implants.52 There were 13 primary procedures and 11 revision procedures (microfracture 7, osteochondral grafting 1, matrix-induced autologous chondrocyte implantation [MACI] 1, and failed fixation osteochondritis dissecans 2). Mean lesion size was 1.8 cm2, 14 of which were on the medial femoral condyle, 4 on the lateral femoral condyle, and 6 on the lateral or central trochlea. Up to four implants were used in the repair. After surgery, patients who had undergone repair on the weight-bearing femoral condyle surface were allowed partial weight bearing at 2 weeks and full weight bearing at 4 weeks.
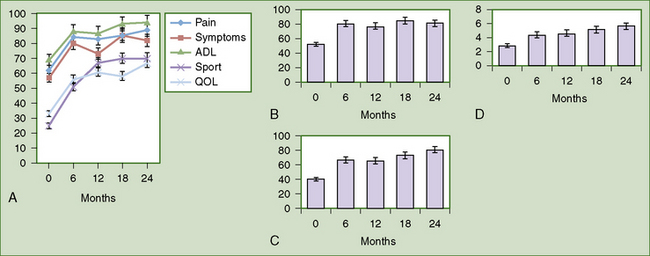
Lysholm score, International Knee Documentation Committee (IKDC) score, and Knee Injury and Osteoarthritis Outcome Score (KOOS) showed statistically significant improvement (P < .001) at 12 months compared to preoperatively (Figure 14–13). Activity levels and hence the mean Tegner activity score (P = .05, see Figure 14–4) improved after 12 months when return to sport was allowed. MRI showed maintenance of the thickness of the new articular surface, gradual differentiation of the implant, and remodeling of the bony component over time (Figure 14–14). Subchondral lamina formation also was evident. Five patients underwent second-look arthroscopy, which showed that although TRUFIT CB stayed soft up to 8 months or more, excellent defect fill and integration with surrounding cartilage were seen. In one patient with slow incorporation of the plugs, patience and perseverance with rehabilitation allowed full resolution of symptoms and return to semiprofessional soccer.53
Following the treatment of chondral or osteochondral lesions in the knee with TRUFIT CB, the most active patients in the series returned to semiprofessional sports; the remainder returned to their previous level of activity by 12 months, with no deterioration to date. This series of patients demonstrated outcome comparable to microfracture at 12 months. The improvement of KOOS (see Figure 14–13) was similar to Saris’ report on patients who received microfracture at 12 months (mean KOOS pain, symptom, activities of daily living [ADL], and quality-of-life [QOL] scores increased 13, 11, 12, and 16 points, respectively, from baseline).54 TRUFIT CB provides a stable scaffold for early mobilization of the knee postoperatively, avoiding the prolonged period of limited weight bearing in microfracture. Other benefits of TRUFIT CB include its availability as an “off-the-shelf” product, the avoidance of donor site morbidity associated with osteochondral grafting, and the ease of the procedure. The results from this case series indicate that TRUFIT CB provides a one-step solution in managing small (<2-cm diameter) areas of chondral or osteochondral injury, particularly in patients who require rapid rehabilitation and a high level of activity.