Abstract
Objective
Previous studies have shown that a customized biomechanical therapy can improve symptoms of knee osteoarthritis. These studies were small and did not compare the improvements across gender, age, BMI or initial severity of knee osteoarthritis. The purpose of this study was to evaluate the effect of new biomechanical therapy on the pain, function and quality of life of patients with medial compartment knee osteoarthritis.
Methods
Six hundred and fifty-four patients with medial compartment knee osteoarthritis were examined before and after 12 weeks of a personalized biomechanical therapy (AposTherapy). Patients were evaluated using the Western Ontario and McMaster Osteoarthritis (WOMAC) Index and SF-36 Health Survey.
Results
After 12 weeks of treatment, the WOMAC-pain and WOMAC-function subscales were significantly lower compared to baseline (both P ≤ 0.001). All eight categories of the SF-36 health survey significantly improved after treatment (all P ≤ 0.001). Females and younger patients showed greater improvements with therapy.
Conclusions
Twelve weeks of a customized biomechanical therapy (AposTherapy) improved symptoms of patients with medial compartment knee osteoarthritis. We recommend that this therapy will be integrated in the management of knee osteoarthritis.
Résumé
Objectifs
Des études ont montré qu’une thérapie biomécanique adaptée pouvait améliorer les symptômes de gonarthrose. À ce jour, toutes les études publiées sur cette nouvelle thérapeutique concernaient des petits échantillons de patients et ne comparaient pas les améliorations en fonction de l’âge, sexe, IMC ou la sévérité initiale de la gonarthrose. Le but de cette étude était d’évaluer l’impact de cette nouvelle thérapie biomécanique sur la douleur, capacité fonctionnelle et qualité de vie des patients avec une gonarthrose du compartiment fémorotibial interne.
Méthodes
Six cent cinquante-quatre patients avec une gonarthrose du compartiment fémorotibial interne étaient suivis avant et après 12 semaines d’un programme thérapeutique biomécanique spécifique (AposTherapy). Les patients étaient évalués avec l’index Western Ontario and McMaster Osteoarthritis (WOMAC) et le questionnaire généraliste de santé SF-36.
Résultats
Après 12 semaines de traitement, les scores du WOMAC-douleur et du WOMAC-capacité avaient diminué de façon significative en comparaison avec les données initiales (les deux p ≤ 0,001). Les huit catégories du SF-36 étaient considérablement améliorées après traitement (toutes p ≤ 0,001). Les femmes et les patients jeunes ont montré un niveau d’amélioration plus important après le traitement.
Conclusion
Les patients avec une gonarthrose du compartiment fémorotibial interne montrent une amélioration de leurs symptômes après 12 semaines de thérapie spécifique AposTherapy. Nous recommandons que cette thérapeutique soit intégrée dans la prise en charge de la gonarthrose.
1
English version
1.1
Introduction
Knee osteoarthritis (OA) is associated with symptoms of pain and functional disability. Physical disability arising from pain and loss of functional capacity reduces quality of life and increases the risk of further morbidity. Current treatments aim at alleviating these symptoms, and some of them address biomechanical factors as well.
Several biomechanical interventions for the treatment of knee OA have been presented. The aims of these interventions are to reduce pain and improve function in patients with knee OA. These interventions attempt to unload the diseased articular surface and in some cases also promote controlled perturbation to train neuromuscular control, usually by means of wedge insoles, foot orthoses and valgus braces . One such biomechanical intervention (AposTherapy) has been presented. In recent studies, Elbaz et al. and Bar-ziv et al. found that patients with knee OA who completed an 8-week biomechanical exercise program reported significant improvements in the level of pain and function, as well as improvement in spatiotemporal gait parameters. The effect of this device on knee adduction moment (KAM), which is highly associated with knee OA, and muscle activation pattern, has also been reported in several studies . To date, all published papers regarding this new therapy were carried out on a relatively small sample size. Furthermore, there is yet to be published a randomized clinical trial regarding the effect of this therapy.
Knee OA is twice as common in women as in men, and usually occurs bilaterally . In end stage knee OA, women undergo almost twice as many knee joint replacement surgeries as men . The fact that men and women have similar rates of occurrence of OA at the hip , argues somewhat against a systemic factor such as estrogen, and in favor of local biomechanical factors as the root cause of knee OA. Although certain gender differences in knee joint anatomy and in biomechanical characteristics have been described , there is a lack of information comparing the effect of knee OA treatments in males and females, especially in the case of biomechanical treatments.
Knee OA is also much more prevalent in the elderly, specifically individuals 60 years of age and older . A couple of studies have shown that knee OA prevalence is even greater in patients 85 years of age and older . A study by Elbaz et al. showed that mainly functional symptoms of knee OA worsen with age . Fewer studies, however, have examined if and how biomechanical therapies for knee OA work in different age groups.
The current study was conducted on a large cohort of patients with medial compartment knee OA and was designed to examine the effect of therapy on gender, age and Body Mass Index (BMI) groups, as well as across the initial symptomatic severity of patients.
1.2
Patients and methods
1.2.1
Participants
This was a retrospective study. A search for eligible data was performed on the research database of AposTherapy Center. Five thousand six hundred and eighty-two people began AposTherapy between April 2009 and September 2010. Three thousand five hundred and twenty-nine patients did not have primary bilateral knee OA. One thousand one hundred and forty-eight patients did not have questionnaires at either baseline or following 3 months of therapy. Three hundred and fifty-one patients were excluded based on the exclusion criteria. Six hundred and fifty-four patients, 446 females (68.2%) and 208 males (31.8%), diagnosed with symptomatic bilateral medial compartment knee OA participated in this study. Two hundred and eighty-nine (44%) patients reported both legs to be equally symptomatic, 209 (32%) patients reported their right leg to be more symptomatic and 156 (24%) patients reported their left leg to be more symptomatic. Mean age (mean ± SD) was 64.7 ± 8.9 years, mean height was 162.3 ± 9.1 cm and mean weight was 84.4 ± 31.3 kg.
Inclusion criteria included:
- •
patients who were examined by their personal physician in the community with bilateral medial compartment knee OA for at least 6 months and who fulfilled the American College of Rheumatology (ACR) clinical criteria for OA of the knee , and were referred to the therapy center. All patients, each of whose charts were reviewed in the present study, were referred to our clinic (AposTherapy center) by their personal physician after being diagnosed with knee OA. This diagnosis was established via a thorough evaluation of the patients by the physician and included a clinical examination and radiographic assessment. When arriving to the therapy center, patients are already diagnosed with radiological knee OA (i.e. radiographs presented signs of OA and no signs of conditions which can mimic OA were present);
- •
patients who completed the Western Ontario and McMaster Osteoarthritis Index (WOMAC) questionnaire and Short Form Health Survey (SF-36) at the start of therapy (study baseline) and after 12 weeks of therapy.
Exclusion criteria included:
- •
neurological and rheumatic inflammatory diseases;
- •
corticosteroid injection within 3 months of the study;
- •
earlier knee surgery excluding arthroscopy;
- •
joint replacement of the hip or knee;
- •
instability of the knee due to traumatic ligament injury;
- •
significant OA in other lower extremity joints.
The protocol was approved by the Institutional Helsinki Committee Registry of Assaf Harofeh Medical Center, Zerifin, Israel (Helsinki registration number 141/08 and NIH clinical trial registration number NCT00767780 ).
1.2.2
Treatment device
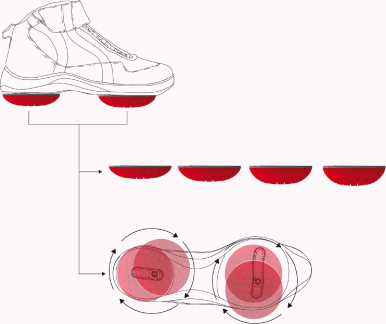
A novel biomechanical device (Apos System, APOS—Medical and Sports Technologies Ltd. Herzliya, Israel) comprised of convex adjustable pods placed under the hind-foot and fore-foot regions of each foot was used. This device enables customize calibration of the pods (i.e. biomechanical elements) which allow for control of body alignment and promotion of perturbation throughout all phases of the step-cycle ( Fig. 1 ).
1.2.3
Pain, function and quality of life analysis
Changes in pain, function and quality of life perception were evaluated using the WOMAC questionnaire and SF-36 Health Survey. The WOMAC questionnaire is a visual analogue scale (VAS) ranging from 0 to 100 mm, with 0 mm indicating no pain or limitation in function and 100 mm indicating the most severe pain or limitation in function. The SF-36 is scored between 0 and 100, with 0 indicating the worst quality of life and 100 indicating the best quality of life.
1.2.4
Study protocol
Prior to their first and second examinations, patients were instructed not to consume pain medication for at least 72 hours in order to eliminate the effect of these medications on the patient’s pain levels. Anthropometric measurements were drawn from the medical file of the patients. All patients completed the WOMAC questionnaire and the SF-36 health survey during their first visit to the therapy center. Patients were guided to complete the WOMAC questionnaire based on the overall condition of their knees. Patients were also guided to complete the SF-36 questionnaire based on their overall health condition. After the completion of the baseline measurements, the biomechanical device was individually calibrated to each patient by a physiotherapist certified in AposTherapy methodology. The principle of calibration is to bring each patient’s joint to a position that allows for diminished pain while walking. In medial compartment knee OA, as was the case for all our patients, the element under the hind-foot is shifted laterally from the baseline position. This is done until the patient reports minimal pain during initial contact. The element under the forefoot is shifted medially from the baseline position until the patient reports minimal pain during mid-stance to toe-off. Biomechanically, by shifting the elements in the coronal plain, the device changes the foot’s COP during gait, thus altering the orientation of the ground reaction force (GRF) vector and reducing the knee adduction forces during gait as well. This decreases the pressure load from the affected area (medial compartment) in the joint during gait .
Once the desired alignment is achieved, the patient usually reports immediate pain relief while walking. All patients received exercise instructions and began the therapy the day after the first visit to the therapy center. Treatment was then initiated and continued on a daily basis for a period of 12 weeks. Patients were instructed to put on the biomechanical device and go about their activities of daily living (ADL) for 10 min once a day during the first week, and gradually increase to 30 min once a day at the fourth week and for the rest of the treatment period. Patients returned for a follow-up examination after 3–4 weeks from initial consultation. If necessary, the Apos system was recalibrated. The patient then continued with the therapy in his or her own personal environment according to the physiotherapist’s instructions. After 12 weeks of treatment, patients underwent a second WOMAC questionnaire and SF-36 Health Survey.
1.2.5
Statistical analysis
With a sample size of 250, the study will have power of 80% to yield a statistically significant result. This computation assumes that the mean difference is at least 5.0 and the common within-group standard deviation is 20.0. This effect (difference of 5 points in each scale) was selected as the smallest effect that would be important to detect, in the sense that any smaller effect would not be of clinical or substantive significance. Mean and standard deviation for all the continuous variables and the mean differences with 95% confidence intervals (CI) were presented for all the continuous variables. The distributions of the questionnaire scales were examined using the Kolmogorov-Smirnov non-parametric test. Paired t -tests were calculated to assess the differences between the repeated measures of the questionnaire scales during follow-up. Tests were also conducted for sub-group analysis. The relationships between the baseline measurements and the improvements level during follow-up were assessed by the Spearman correlation. Data were analyzed with SPSS software version 19.0. The significance levels were set at 0.05.
1.3
Results
Baseline levels of pain, function, stiffness and quality of life are presented in Table 1 . There were no reports of any adverse events including imbalance, tripping or other physical problems during the study period. All patients complied completely with the treatment protocol. Compliance was verified via a telephone call at several points during the study.
| Baseline | 3 months | Mean difference | 95% CI of the difference-Lower bound | 95% CI of the difference-Upper bound | P * | |
|---|---|---|---|---|---|---|
| WOMAC Index a | ||||||
| Pain | 50.1 (20.0) | 35.0 (21.1) | 15.0 (21.0) | 13.4 | 16.7 | < 0.001 |
| Stiffness | 51.0 (27.2) | 36.7 (26.2) | 14.4 (17.8) | 12.4 | 16.5 | < 0.001 |
| Function | 48.7 (19.2) | 34.6 (20.3) | 14.1 (17.8) | 12.7 | 15.5 | < 0.001 |
| SF-36 Health Survey b | ||||||
| Physical function | 46.5 (20.7) | 51.2 (22.1) | 4.7 (18.4) | 6.1 | 3.3 | < 0.001 |
| Pain | 41.6 (21.6) | 53.8 (22.2) | 12.2 (23.7) | 14.0 | 10.3 | < 0.001 |
| Role limitation due to physical health | 36.4 (36.9) | 49.1 (39.1) | 12.7 (40.2) | 15.8 | 9.7 | < 0.001 |
| Energy/Fatigue | 53.7 (19.5) | 57.0 (17.6) | 3.3 (17.2) | 4.6 | 1.9 | < 0.001 |
| Emotional well-being | 67.8 (18.3) | 72.0 (16.5) | 4.2 (16.3) | 5.4 | 2.9 | < 0.001 |
| Role limitation due to emotional health | 53.8 (43.2) | 65.8 (40.6) | 12.0 (46.5) | 15.6 | 8.5 | < 0.001 |
| Social functioning | 68.7 (25.9) | 75.5 (23.3) | 6.8 (25.7) | 8.8 | 4.8 | < 0.001 |
| General health | 58.6 (17.0) | 61.9 (17.3) | 3.3 (15.2) | 4.4 | 2.1 | < 0.001 |
| SF-36 physical scale | 47.4 (17.2) | 54.6 (18.2) | 13.2 (11.5) | 14.1 | 12.3 | < 0.001 |
| SF-36 mental scale | 60.5 (18.9) | 66.4 (17.9) | 11.9 (10.7) | 12.7 | 11.0 | < 0.001 |
a Western Ontario and McMaster Universities Index (WOMAC Index). The WOMAC questionnaire includes 24 questions in a Visual Analogue Scale (VAS) format (0 = no pain/stiffness/difficulty, 100 = severe pain/stiffness/difficulty). The criteria for clinical response to a treatment had been defined by the Outcome Measures in Rheumatology Clinical Trials (OMERACT) and Osteoarthritis Research Society International (OARSI). They are either an improvement in pain or in function of at least 50% with a decrease of 2.0 cm on the VAS for pain or function, or an improvement in both pain and function of at least 20% with a decrease of 1.0 cm on the VAS. The average improvements in the WOMAC pain and function scales meet the OMERACT-OARSI criteria. Furthermore, individually, 56% of the patients met the OMERACT-OARSI criteria (improved) whereas 18% of the patients improved but did not meet these criteria.
b SF-36 Health Survey includes 36 questions. Results range between 0–100 (0 = poor quality of life, 100 = high quality of life).
After 12 weeks of treatment, the WOMAC-pain and WOMAC-function subscales were significantly lower compared to baseline ( Table 1 ). Pain decreased by 30% ( P ≤ 0.001) and function improved by 29% ( P ≤ 0.001). All eight categories of the SF-36 health survey significantly improved after 12 weeks of treatment ( Table 1 ).
To find the specific effect of the treatment in sub-groups, a further analysis on the WOMAC and SF-36 overall score was carried out. Significant differences between genders were found at baseline in the WOMAC-pain, WOMAC-stiffness and WOMAC-function categories. Females reported higher levels of pain (11.6%), stiffness (15.3%) and functional limitation (7.1%). No significant gender differences were seen in the eight categories of the SF-36 at baseline. Both males and females showed significant improvement in all WOMAC and SF-36 subcategories following 12 weeks of therapy. Results are summarized in Table 2 .
| Females | Males | P † | P †† | P ††† | P †††† | |||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | |||||
| WOMAC Index a | ||||||||
| Pain | 51.9 (20.0) | 36.1 (21.3) | 46.2 (19.6) | 32.9 (20.5) | 0.001 | NS | < 0.001 | < 0.001 |
| Stiffness | 53.5 (26.8) | 37.8 (26.2) | 45.9 (27.3) | 34.2 (26.1) | 0.001 | NS | < 0.001 | < 0.001 |
| Function | 49.8 (19.5) | 35.5 (20.0) | 46.4 (18.3) | 32.8 (20.8) | 0.03 | NS | < 0.001 | < 0.001 |
| SF-36 Health Survey b | ||||||||
| Physical function | 47.2 (20.9) | 52.1 (22.4) | 45.0 (20.3) | 49.4 (21.6) | NS | NS | < 0.001 | 0.002 |
| Pain | 41.6 (21.1) | 54.3 (22.0) | 41.7 (22.7) | 52.6 (22.8) | NS | NS | < 0.001 | < 0.001 |
| Role limitation due to physical health | 38.0 (37.6) | 49.9 (39.1) | 32.9 (35.2) | 47.3 (39.1) | NS | NS | < 0.001 | < 0.001 |
| Energy/Fatigue | 53.3 (19.7) | 57.0 (18.5) | 54.6 (19.0) | 56.8 (15.4) | NS | NS | < 0.001 | NS |
| Emotional well-being | 67.8 (18.8) | 72.1 (16.5) | 68.0 (17.1) | 71.9 (16.6) | NS | NS | < 0.001 | 0.003 |
| Role limitation due to emotional health | 55.5 (42.8) | 66.6 (40.7) | 50.1 (44.0) | 64.3 (40.5) | NS | NS | < 0.001 | < 0.001 |
| Social functioning | 69.1 (25.7) | 75.8 (23.5) | 67.9 (26.3) | 74.8 (22.8) | NS | NS | < 0.001 | < 0.001 |
| General health | 58.8 (17.6) | 61.9 (17.7) | 58.2 (15.8) | 62.0 (16.6) | NS | NS | < 0.001 | < 0.001 |
| SF-36 physical scale | 47.8 (17.4) | 55.0 (18.5) | 46.5 (16.8) | 53.6 (17.5) | NS | NS | < 0.001 | < 0.001 |
| SF-36 mental scale | 60.9 (19.3) | 66.7 (18.4) | 59.8 (18.0) | 65.9 (16.9) | NS | NS | < 0.001 | < 0.001 |
a Western Ontario and McMaster Universities Index (WOMAC Index). The WOMAC questionnaire includes 24 questions in a VAS format (0 = no pain/stiffness/difficulty, 100 = severe pain/stiffness/difficulty).
b SF-36 Health Survey includes 36 questions. Results range between 0–100 (0 = poor quality of life, 100 = high quality of life).
Age was divided based on the median age (66 years). There were significant differences in the baseline values between the two age groups in WOMAC-pain and WOMAC-stiffness. The younger group (< 66 years) reported higher levels of pain (10%) and functional limitation (15.8%) compared to the older group (> 66 years). The WOMAC-function and all SF-36 categories were not significantly different between the two groups at baseline. Both age groups showed significant improvement in all WOMAC and SF-36 categories following 12 weeks of therapy. Results are summarized in Table 3 .
| < 66 | ≥66 | P † | P †† | P ††† | P †††† | |||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | |||||
| WOMAC Index a | ||||||||
| Pain | 52.6 (20.2) | 34.5 (21.5) | 47.4 (19.3) | 35.0 (20.6) | 0.002 | NS | < 0.001 | < 0.001 |
| Stiffness | 53.8 (27.5) | 37.0 (25.6) | 45.9 (27.3) | 34.2 (26.1) | 0.02 | NS | < 0.001 | < 0.001 |
| Function | 49.9 (20.0) | 34.2 (21.3) | 47.6 (18.3) | 35.0 (20.2) | NS | NS | < 0.001 | < 0.001 |
| SF-36 Health Survey b | ||||||||
| Physical function | 46.5 (21.0) | 52.1 (22.2) | 46.0 (20.6) | 49.8 (21.9) | NS | NS | < 0.001 | < 0.001 |
| Pain | 41.5 (20.5) | 52.3 (22.2) | 42.4 (22.2) | 55.2 (22.6) | NS | NS | < 0.001 | < 0.001 |
| Role limitation due to physical health | 35.4 (36.9) | 47.5 (38.8) | 38.2 (36.7) | 50.6 (39.5) | NS | NS | < 0.001 | < 0.001 |
| Energy/Fatigue | 54.3 (19.4) | 57.3 (18.3) | 53.6 (19.3) | 57.2 (16.4) | NS | NS | 0.004 | 0.001 |
| Emotional well-being | 67.7 (17.9) | 72.1 (16.2) | 68.4 (18.9) | 72.7 (16.9) | NS | NS | < 0.001 | < 0.001 |
| Role limitation due to emotional health | 53.2 (42.1) | 66.4 (39.9) | 54.3 (44.1) | 65.4 (41.9) | NS | NS | < 0.001 | < 0.001 |
| Social functioning | 67.9 (25.8) | 74.7 (23.9) | 70.3 (26.3) | 76.9 (23.1) | NS | NS | < 0.001 | < 0.001 |
| General health | 58.5 (17.8) | 61.5 (18.0) | 58.9 (16.5) | 62.4 (17.4) | NS | NS | 0.002 | < 0.001 |
| SF-36 physical scale | 47.2 (17.6) | 54.1 (18.3) | 47.8 (16.7) | 55.0 (18.1) | NS | NS | < 0.001 | < 0.001 |
| SF-36 mental scale | 60.3 (18.9) | 66.4 (17.9) | 61.1 (19.1) | 66.9 (18.4) | NS | NS | < 0.001 | < 0.001 |
a Western Ontario and McMaster Universities Index (WOMAC Index). The WOMAC questionnaire includes 24 questions in a VAS format (0 = no pain/stiffness/difficulty, 100 = severe pain/stiffness/difficulty).
b SF-36 Health Survey includes 36 questions. Results range between 0–100 (0 = poor quality of life, 100 = high quality of life).
Another examination was carried out according to the patients’ BMI. Two groups of patients were defined: patients with a BMI less or equal to 28.0 kg/m 2 and patients with a BMI greater than 28.0 kg/m 2 . There were significant differences in the baseline values between the two groups in WOMAC-pain, WOMAC-stiffness and WOMAC-function. The lighter weight group (BMI ≤ 28.0 kg/m 2 ) reported lower levels of pain (9.3%), stiffness (12.4%) and functional limitation (15.0%) compared to the heavier weight group (BMI > 28.0 kg/m 2 ). All SF-36 categories were not significantly different between the two groups at baseline. After 3 months of therapy there were significant differences between groups in WOMAC-function. Both BMI groups showed significant improvement in all WOMAC and SF-36 categories following 12 weeks of therapy, except for the SF-36 energy subscale in which the lighter BMI group did not improve significantly. Results are summarized in Table 4 .
| BMI ≤ 28.0 | BMI > 28.0 | P † | P †† | P ††† | P †††† | |||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | |||||
| WOMAC Index a | ||||||||
| Pain | 46.9 (19.5) | 32.8 (22.1) | 51.5 (20.1) | 35.6 (20.4) | 0.012 | NS | < 0.001 | < 0.001 |
| Stiffness | 46.9 (27.2) | 35.3 (28.0) | 53.1 (27.0) | 37.4 (25.2) | 0.012 | NS | < 0.001 | < 0.001 |
| Function | 43.8 (18.8) | 30.8 (20.5) | 50.9 (18.9) | 36.3 (20.3) | < 0.001 | 0.004 | < 0.001 | < 0.001 |
| SF-36 Health Survey b | ||||||||
| Physical function | 47.1 (22.0) | 50.4 (22.3) | 45.9 (20.5) | 51.4 (22.0) | NS | NS | 0.024 | < 0.001 |
| Pain | 42.4 (21.0) | 53.1 (23.6) | 41.9 (21.4) | 53.9 (21.7) | NS | NS | < 0.001 | < 0.001 |
| Role limitation due to physical health | 38.8 (36.8) | 49.3 (40.1) | 36.1 (36.9) | 48.4 (39.0) | NS | NS | 0.002 | < 0.001 |
| Energy/Fatigue | 53.1 (19.4) | 55.4 (18.0) | 54.6 (19.2) | 57.9 (17.0) | NS | NS | NS | < 0.001 |
| Emotional well-being | 67.8 (18.6) | 71.6 (16.2) | 68.3 (18.1) | 73.0 (16.3) | NS | NS | 0.003 | < 0.001 |
| Role limitation due to emotional health | 57.8 (42.8) | 67.6 (41.1) | 52.5 (43.1) | 65.5 (40.3) | NS | NS | 0.007 | < 0.001 |
| Social functioning | 69.6 (25.8) | 74.6 (25.5) | 68.8 (26.1) | 76.5 (22.2) | NS | NS | 0.023 | < 0.001 |
| General health | 60.4 (17.1) | 63.1 (18.4) | 57.9 (17.1) | 61.4 (17.3) | NS | NS | 0.03 | < 0.001 |
| SF-36 physical scale | 48.4 (17.3) | 54.3 (19.7) | 47.3 (17.2) | 54.6 (18.5) | NS | NS | < 0.001 | < 0.001 |
| SF-36 mental scale | 61.7 (18.5) | 66.5 (18.9) | 60.4 (19.1) | 66.9 (17.6) | NS | NS | < 0.001 | < 0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







