Developmental Defects
Marvin A. Fishman
HYDROCEPHALUS
Hydrocephalus is a congenital or acquired disorder in which an excessive amount of cerebrospinal fluid (CSF) is present within the cerebral ventricles. More CSF is produced than can be reabsorbed. Increased pressure within the ventricular system may be transitory or persistent. Enlargement of cerebral ventricles caused by the loss of brain tissue (formerly called hydrocephalus ex vacuo) is excluded from consideration in this chapter because it does not meet the definition of inadequate absorption of CSF and increased pressure. Noncommunicating hydrocephalus refers to conditions in which the ventricular fluid does not communicate with the fluid in the basal cisterns or spinal subarachnoid spaces. It implies a block of the flow of CSF within the ventricular system. In communicating hydrocephalus, the block is outside the ventricular system or its exit foramina.
CSF is formed within the ventricular system, mainly by the choroid plexus, through the processes of active secretion and diffusion. The fluid exits the ventricular system by way of foramina in the fourth ventricle and circulates into the lumbar and subarachnoid spaces. Most absorption of CSF takes place at the arachnoid villi leading to venous channels of the sagittal sinus. In adults, the total volume of CSF is approximately 150 mL, and only 25% is within the ventricular system. The rate of formation is approximately 20 mL/hour, and the CSF turns over three to four times/day. Production of CSF in children appears to be related to age and body weight. Formation appears to increase rapidly in infancy and by the time the child is 2 years of age is approximately 60% of that at 15 years of age.
Pathogenesis
Impaired absorption of CSF resulting from obstruction of flow or dysfunction of absorptive mechanisms is the most common mechanism for producing hydrocephalus. If flow is blocked within the ventriclar system, a disproportionate dilatation of the ventricles proximal to the block occurs. In aqueductal stenosis, the lateral and third ventricles are dilated disproportionately compared with the fourth ventricle. If the block is extraventricular, a relatively proportionate increase in the sizes of all ventricles occurs. Thrombosis of the superior sagittal sinus can interfere with absorption of the CSF, but, with venous sinus thrombosis, cerebral edema or infarction of the cerebral cortex usually is present; hydrocephalus occurs only late in the illness.
Hydrocephalus secondary to excessive secretion of CSF is a rare development; when it occurs, it usually is associated with a functioning choroid plexus papilloma. Mechanical obstruction by the tumor or fibrosis of the subarachnoid spaces secondary to bleeding from the tumor may contribute to the hydrocephalus.
With obstruction of the flow of CSF, compensatory mechanisms of absorption may develop. One of these is increased movement of CSF across the ependymal lining of the ventricular system, which usually occurs when a significant increase in CSF pressure is present; it is detected as periventricular areas of low density by computed tomography (CT) or as increased periventricular water by magnetic resonance imaging (MRI). Ventricular dilatation usually begins in the frontal and occipital horns of the lateral ventricles and is followed by more symmetric dilatation of the remainder of the ventricles. In patients with an intraventricular block, the subarachnoid space over the cerebral hemispheres becomes obliterated and the vascular system becomes compressed; as a result, venous pressure in the dural sinuses increases. Eventually, the cerebral mantle becomes thinner to accommodate the increased volume of CSF. If the cranial sutures have not fused, abnormal increases in head size occur in infants. In older children, this compensatory mechanism is not available; intracranial pressure (ICP) increases, and symptoms develop more readily than in young infants. The rapidity and severity of the process depend on the imbalance between production and absorption of CSF. The greater the imbalance, the faster symptoms and signs develop and the more severe they are. Complete obstruction to absorption is not compatible with life. With only a slight imbalance between production and absorption, the process is slower and more insidious. The imbalance may become compensated as alternative mechanisms of CSF absorption develop but at the expense of increased ventricular size.
Etiology
Congenital hydrocephalus may result from congenital malformations of the nervous system, including isolated aqueductal stenosis, or it may be associated with other malformations, including the Dandy-Walker malformation. The latter consists of a large cyst in the posterior fossa continuous with the fourth ventricle and partial or complete absence of the cerebellar vermis. A common malformation syndrome is that of meningomyelocele with Chiari malformation. Other syndromes include a sex-linked form of aqueductal stenosis. Other central nervous system anomalies may be present, including dysgenesis of the corpus callosum, small brainstem, and absence of the pyramidal tract. The infants often have adducted thumbs. The disorder is caused by a mutation in the gene encoding L 1, a neuronal cell adhesion molecule, and it is mapped to Xq28. Chromosomal abnormalities (trisomies 13, 18, 19, 9, and 9p) resulting in syndromes with additional multiple congenital malformations are associated with congenital hydrocephalus. Arachnoid cysts or congenital tumors may obstruct the ventricular system. Congenital hydrocephalus may result from intrauterine infections, which cause inflammation of the ependymal lining of the ventricular system or the meninges in the subarachnoid space, thus subsequently occluding the CSF pathways. Among the more common infections causing congenital hydrocephalus are rubella, cytomegalovirus infection, toxoplasmosis, and syphilis.
Hydrocephalus may be acquired postnatally secondary to infections of the nervous system (e.g., bacterial meningitis),
brain tumors, and arachnoiditis caused by bleeding into the subarachnoid space from a ruptured arteriovenous malformation, aneurysm, or trauma. Premature infants may develop hydrocephalus secondary to intraventricular hemorrhage.
brain tumors, and arachnoiditis caused by bleeding into the subarachnoid space from a ruptured arteriovenous malformation, aneurysm, or trauma. Premature infants may develop hydrocephalus secondary to intraventricular hemorrhage.
Symptoms and Signs
The primary process (e.g., tumor, infection, bleeding) and the symptom and signs caused by increased ICP secondary to the hydrocephalus may contribute to the clinical picture. The severity of the findings is influenced by the rate at which the hydrocephalus develops and the development of alternate pathways of absorption of CSF. Nonspecific symptoms include headaches that are variable in location and intensity; they occasionally occur early in the morning and are associated with vomiting. Personality and behavior changes, including irritability or indifference, sometimes occur. Lethargy and drowsiness are relatively late symptoms. Nausea and vomiting are secondary to increased ICP, particularly with posterior fossa lesions. Nonspecific signs include third and sixth cranial nerve deficits, which result in paresis of extraocular muscles and may lead to diplopia. Papilledema may be a late finding if the ICP is not markedly elevated and the process is slow and chronic. Changes in vital signs occur relatively late and indicate distortion of the brainstem. In young children, the anterior fontanelle may become full or distended; this condition is accompanied by excessive head growth and dilatation of scalp veins. The “setting-sun sign” is produced by paralysis of upward gaze so the sclera above the iris is visible. Spasticity develops first in the lower extremities and then in the arms and results from stretching of motor fibers around the bodies of the lateral ventricles. Dilatation of the third ventricle may cause pressure on the hypothalamus, resulting in disturbances in sexual development and in fluid and electrolyte imbalances. Specific deficits produced by focal lesions include hemiparesis, ataxia, tremor, speech and language disorders, gaze disorders, facial weakness, and difficulty in swallowing. Seizures are unusual isolated presenting symptoms of hydrocephalus and often are caused by the underlying associated condition.
Diagnosis and Therapy
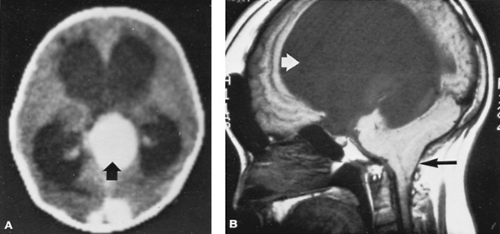
Neuroimaging techniques such as CT or MRI have made the diagnosis of hydrocephalus relatively straightforward. The pattern of ventricular dilatation, the presence of interstitial edema (i.e., CSF in the white matter surrounding the ventricles), and an underlying cause for obstruction of CSF flow usually are readily apparent (Fig. 395.1). The CSF should be examined if a relatively recent infection is suspected or if subarachnoid bleeding is suspected but no evidence of such is found on neuroimaging studies. In infancy, chronic subdural hematomas may present in a similar fashion and can be detected by neuroimaging procedures.
Treatment includes specific therapy for any underlying condition associated with the hydrocephalus, such as brain tumor, abscess, and chronic meningitis. Surgery is the most effective means of treating progressive hydrocephalus; a shunt system is placed between the cerebral ventricles and the peritoneal cavity. Shunt placement is a palliative measure and not a cure. The complication rate is relatively high. Problems encountered include mechanical obstruction of the shunt system. The failure rate may be as high as 40% in the first year. Infections within the shunt system may produce meningitis or ventriculitis. Shunt infections may be indolent and often are caused by organisms that usually are not considered pathogens, such as Staphylococcus epidermidis. Other surgical procedures include third ventriculotomies in certain conditions. Shunt infections occur in approximately 5% to 10% of procedures. Most occur within the first 6 months of placement of the shunt. Medical therapy designed to decrease production of CSF may be used when the hydrocephalus is slowly progressive and perhaps transitory. Such conditions include the ventricular enlargement sometimes seen after subarachnoid hemorrhage or meningitis. The therapeutic agents used include acetazolamide, furosemide, and glycerol. Diuretic therapy in newborns with posthemorrhage ventricular dilatation does not appear to be effective in reducing the need for placement of a shunt system. These agents also may be used in the interim between the removal of an infected shunt system and insertion of a new system. Serial lumbar punctures in preterm infants with posthemorrhagic ventriculomegaly do
not appear to be effective in reducing the risk for shunt placement, death, or disability.
not appear to be effective in reducing the risk for shunt placement, death, or disability.