Crohn Disease
W. Daniel Jackson
Stephen L. Guthery
Richard J. Grand
Crohn disease (CD) is a transmural inflammatory process that may affect any segment of the gastrointestinal (GI) tract from mouth to anus in a discontinuous fashion. The small bowel is involved in 90% of cases, predominantly the distal ileum (70%), usually in combination with colitis as ileocolitis (more than 50%). Isolated colonic disease without clinical or radiologic evidence of small bowel involvement occurs in approximately 10% of patients. The small bowel involvement is responsible for many of the specific nutritional complications of CD, whereas the colonic involvement poses the greatest challenge for differentiation from other infectious and inflammatory bowel diseases. Although CD shares many features with ulcerative colitis (UC), several features allow differentiation of the two disorders (Table 352.1). This chapter, therefore, complements Chapter 352.
PATHOLOGY
Unlike the findings in UC, the inflammation in CD usually does not involve a continuous segment of bowel and often appears as discrete focal ulcerations (e.g., aphthae) with relatively intact intervening mucosa. As the disease progresses, in the 60% of cases involving the colon, right-sided or proximal colonic inflammation predominates, with relative sparing of the rectum. Anal involvement, in the form of skin tags, anal fissures, abscesses, and fistulas, occurs in approximately 25% of patients, often preceding intestinal symptoms. The inflammation of CD usually is transmural and can be recognized as mesenteric inflammation; fat encroachment on the serosal surface of the intestine; stiffening of the small bowel loops caused by
fibrosis; and adhesions, stricture formation, and fistulas to other loops of bowel, bladder, vagina, or skin.
fibrosis; and adhesions, stricture formation, and fistulas to other loops of bowel, bladder, vagina, or skin.
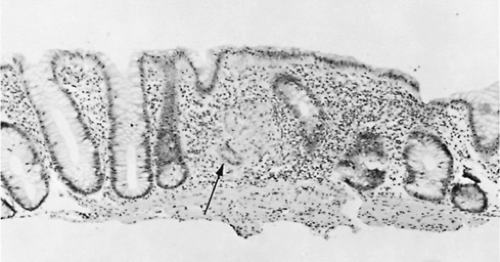
The histology of the lesions in CD reveals the transmural nature of the acute and chronic inflammation, often showing edema, lymphoid aggregates, and significant fibrosis. Mucosal changes may resemble those of ulcerative or infectious colitis characterized by acute polymorphonuclear leukocyte invasion of crypt epithelium to cause cryptitis or crypt abscesses, as well as chronic features of crypt branching and dropout distorting normal crypt architecture. Some areas of the bowel may be normal or may show only mild chronic inflammation. Noncaseating granulomas may be found in as many as 50% of the patients, and coupled with the transmural inflammation, disease distribution, and clinical presentation, their presence provides strong support for the diagnosis of CD (Fig. 353.1).
ETIOLOGY
As in UC, the cause of CD is unknown, with evidence existing for the collusion of multiple factors, including genetics, luminal agents, altered mucosal integrity, and immunologic response, in the pathogenesis. A current hypothesis suggests that CD is caused by an inappropriate cellular immune response to enteral bacterial products in a genetically susceptible host. The pathogenesis of the chronic inflammation may involve abnormalities in the regulation of the immune response to infectious, toxic, or dietary-derived intestinal antigens or an inappropriate immune response to an unusual antigen or infectious agent. No consistent pathogen has been identified. Altered colonic flora or bacterial products may play a role in the pathogenesis, given the attenuation or absence of disease in germ-free animal models and the therapeutic usefulness of antibiotics. Impaired intestinal mucosal exclusion of luminal antigens may be a causal factor or simply a consequence of inflammation. Inappropriately regulated immune or cytokine responses have been implicated on the basis of the favorable response to corticosteroids, other immunosuppressive agents, and cytokine antagonists. Although evidence for an activated T-lymphocyte population and cytokine production exists, no specific markers of autoimmunity have been demonstrated.
A genetic basis of CD is supported by several observations. First, the prevalence of CD is higher among some subpopulations (e.g., Ashkenazi Jews). Second, concordance of disease among monozygotic twins is approximately 67%. Third, multiple independent genome-wide scans have revealed susceptibility loci in families with several affected members. Finally, mutations in the CARD15/NOD2 gene on chromosome 16 confer an increased risk among both heterozygotes (two- to fourfold) and homozygotes (40-fold). The normal protein product of CARD15/NOD2 is responsible for binding intracellular bacterial products and transducing intracellular inflammatory signals involved in bacterial recognition by monocytes. Not all individuals with CD have mutations in CARD15/NOD2; conversely, not all those with mutations in CARD15/NOD2 develop CD. These features, as well as the several other identified susceptibility loci (e.g., on chromosomes 5 and 7), suggest that CD has a non-Mendelian complex genetic basis.
EPIDEMIOLOGY
A recent epidemiologic study of children diagnosed by pediatric gastroenterologists in Wisconsin revealed an incidence of inflammatory bowel disease (IBD) of 7.05 cases per 100,000 per year. The majority of cases, 4.56 per 100,000 population, were CD, more than twice the incidence of UC. In most cases, no family history existed. Unlike previous studies indicating an increased prevalence among whites (especially in the Jewish population) in the developed world, the incidence was not affected by racial or urbanization factors. Male and female representation is approximately equal, as is bimodal age at onset, with peaks occurring in the second and third and again in the sixth decades of life. Approximately 2% of cases, including rare cases in infancy presenting as intractable diarrhea, occur before the patient reaches 10 years of age.
CLINICAL PRESENTATION
The presentation of CD in children depends on the location and extent of inflammation. In many cases, the onset is insidious with nonspecific features of GI involvement or extraintestinal manifestations leading to delayed or incorrect diagnosis. Diarrhea, abdominal pain (most frequently postprandial periumbilical cramping), fever, and weight loss are the most common presenting features. Rectal bleeding, seen in 30% of CD cases, is seen much less commonly than in UC and signifies colonic involvement. The three general patterns of clinical presentation based on anatomic involvement show considerable overlap: (1) diarrhea and lower abdominal cramping representing colonic inflammation; (2) nausea, vomiting, anorexia, and/or early satiety due to upper gastrointestinal or small intestinal disease; and (3) extraintestinal symptoms and signs, including delayed maturation and growth impairment (Box 353.1).
Colonic involvement may present as diarrhea, often associated with cramps and urgency to defecate after any distention of the inflamed colon by the fecal stream. Other signs of colitis may be indistinguishable from those seen in UC and consist
of an inflammatory exudate of neutrophils into the lumen and occult or overt rectal bleeding. Perianal disease and relative sparing of the rectum occur more frequently in Crohn colitis than in ulcerative colitis and may be the only differentiating features. Perianal skin tags and fissures may be the first physical sign of disease, especially if they are off the sagittal plane. Perianal abscesses and perineal fistulas also should suggest the diagnosis of Crohn colitis. A rare complication, toxic dilation with risk of perforation and sepsis known as toxic megacolon, has been reported in Crohn colitis, though less often than in UC; treatment is the same as that outlined for toxic megacolon complicating severe ulcerative colitis (see Chapter 352).
of an inflammatory exudate of neutrophils into the lumen and occult or overt rectal bleeding. Perianal disease and relative sparing of the rectum occur more frequently in Crohn colitis than in ulcerative colitis and may be the only differentiating features. Perianal skin tags and fissures may be the first physical sign of disease, especially if they are off the sagittal plane. Perianal abscesses and perineal fistulas also should suggest the diagnosis of Crohn colitis. A rare complication, toxic dilation with risk of perforation and sepsis known as toxic megacolon, has been reported in Crohn colitis, though less often than in UC; treatment is the same as that outlined for toxic megacolon complicating severe ulcerative colitis (see Chapter 352).
BOX 353.1 Crohn Disease: Patterns of Involvement
Extraintestinal signs and growth retardation
Anorexia, malaise, fatigue
Perianal disease, oral aphthae
Erythema nodosum, pyoderma gangrenosum
Anemia, hepatitis, nephrolithiasis, arthritis, clubbing
Small bowel involvement
Nausea, early satiety
Abdominal mass, postprandial cramps
Diarrhea, malabsorption
Mineral and vitamin deficiencies (iron, zinc, magnesium, folate, vitamin B12)
Colonic features
Diarrhea, urgency
Rectal bleeding, fecal leukocytes
Perianal fistula, abscess
Another pattern of presentation is produced by upper gastrointestinal inflammation, which probably accounts for much of the postprandial cramping, early satiety, nausea, and anorexia that patients report. Rare involvement of the esophagus or gastroduodenal segments may mimic peptic disease and respond partially to acid control therapy. Gastritis or duodenal ulceration in the absence of Helicobacter pylori infection or use of nonsteroidal antiinflammatory agents should suggest possible CD. Diarrhea may occur in 90% of patients with small bowel involvement and, in the absence of colitis, most likely signifies malabsorption of bile acids by the terminal ileum or nutrients. Estimates of the prevalence of malabsorption in children with CD depend on the nutrient that is malabsorbed but range from 17% for lactose and 29% for fat to 70% for protein. The frequency of lactose malabsorption is normal when adjusted for that expected for the ethnic distribution of patients with CD but in some patients may be a consequence of small bowel inflammation. Deficiencies of iron, zinc, magnesium, folate, and vitamin B12 may be more pronounced in patients with small bowel disease, particularly those with terminal ileum involvement.
Patients may present with nonspecific extraintestinal manifestations and growth retardation (see Box 353.1). Overt clinical signs of involvement of the GI tract may not appear for years, although this inflammation may be extensive enough to cause early satiety, nausea, anorexia, and distention as signs of malabsorption or impaired transit and partial obstruction. Over the course of time, net energy and protein deficits are reflected in decreased weight velocity followed by decreased height velocity and delayed skeletal and sexual maturation. As a consequence of nutritional, growth, and maturational problems, patients may be referred to specialists in endocrinology for assessment of short stature and hypogonadism or to psychiatrists for evaluation of anorexia. Certain extraintestinal features that may be clues indicating the presence of CD include perianal disease, oral aphthae, erythema nodosum, arthritis, uveitis, and digital clubbing. A microcytic anemia with reduced total iron-binding capacity, an elevated leukocyte and platelet count, and an elevated erythrocyte sedimentation rate may be present. Abdominal radiography may show an unusual gas pattern with some small bowel dilation; usually, however, radiography does not establish a diagnosis. Recognizing this insidious mode of presentation leads to timely use of specific tests to confirm the diagnosis.
Extraintestinal Signs
The systemic nature of CD is apparent in the range of potential involvement of extraintestinal organs. Arthritis and arthralgias may occur in as many as 11% of cases and usually present as a seronegative monoarticular arthritis of large joints such as a knee or ankle or as a migratory polyarthritis. Arthritis occurs more commonly in patients with colonic involvement (e.g., colitis, ileocolitis) and seems to parallel the activity of disease, although occasionally it precedes overt gastrointestinal signs. Sacroiliitis and ankylosing spondylitis rarely occur, predominantly in patients with histocompatibility gene HLA-B27.
Fewer than 5% of patients develop cutaneous lesions of erythema nodosum or pyoderma gangrenosum. The latter condition is a severe deep ulceration of the skin, often preceded by minor trauma or associated with surgical incisions or stoma sites. Management requires control of the underlying bowel disease, often with the addition of metronidazole, topical tacrolimus, dapsone, local corticosteroid injection, and occasional skin grafting.
Signs of liver disease occur in fewer than 4% of patients with CD. Mild histologic abnormalities of steatosis may be more common findings. Involvement of the liver correlates with the activity of bowel disease but rarely progresses to cirrhosis or chronic active hepatitis. Sclerosing cholangitis has been reported in two adolescents with Crohn colitis and is not associated exclusively with UC. Cholelithiasis, usually asymptomatic, may occur after ileal dysfunction or resection that interrupts the enterohepatic circulation of bile acids, leading to the decreased cholesterol solubility characteristic of lithogenic bile. Prolonged periods of bowel rest without meal-stimulated contraction of the gallbladder allows stasis of bile in the gallbladder, which contributes to sludge and formation of stones.
Urologic manifestations include calcium oxalate renal calculi caused by increased intestinal oxalate absorption accompanying steatorrhea and subsequent increased renal excretion. Ureteral inflammation may develop from adjacent transmural bowel inflammation and leads to pyuria, obstruction, and infection. Hydroureter or hydronephrosis may result from renal stones, inflammation, or external compression from adjacent intestinal masses. Recurrent urinary tract infections and pneumaturia may herald enterovesical fistulas.
Other signs include uveitis, acutely symptomatic in fewer than 3% and asymptomatic in as many as 30% of patients; aphthous stomatitis; osteoporosis; anemias of chronic disease; iron, vitamin B12, and folate deficiencies; zinc deficiency, implicated when there is taste dysfunction, acrodermatitis, and poor healing; thyroid dysfunction and enlargement; and growth failure.
Undernutrition and Growth Failure
Weight loss occurs in as many as 87% of children presenting with CD. Often accompanying the weight loss are impaired
linear growth, retarded bone development and mineralization, and delayed sexual maturation. These changes may be subtle initially and often precede development of overt bowel disease by months or years. Most of these effects seem to be caused by undernutrition because they can be reversed by nutritional supplementation.
linear growth, retarded bone development and mineralization, and delayed sexual maturation. These changes may be subtle initially and often precede development of overt bowel disease by months or years. Most of these effects seem to be caused by undernutrition because they can be reversed by nutritional supplementation.
The cause of undernutrition in IBD is multifactorial. In most patients with growth failure, dietary energy intake is less than the average requirement for age and may be the result of anorexia from altered taste, early satiety, or meal-related cramps or diarrhea. Some cases are complicated by steatorrhea (29%) and increased enteric protein excretion (70%). Hypoalbuminemia (50%), hypomagnesemia, hypocalcemia, fat-soluble vitamin losses, and deficiencies in iron, folate, vitamin B12, and zinc may be present. Nutrient requirements are increased by the metabolic demands of chronic inflammation, losses through fistulas, and demands for repletion of lean body mass and fat deficits beyond those normally imposed by growth, especially in adolescents.
Most endocrine test results are normal in patients with growth retardation and short stature associated with CD. Although bone age may be delayed and serum insulinlike growth factor-1 levels depressed, both respond to nutritional therapy, and pituitary, thyroid, adrenal, and growth hormone studies typically are normal. One study revealed a high prevalence of thyroidomegaly and hypothyroidism in adults with CD. Sexual maturation, arrested by disease and nutrient deficits leading to delayed puberty and menarche, resumes after nutritional therapy is initiated and inflammation is controlled.
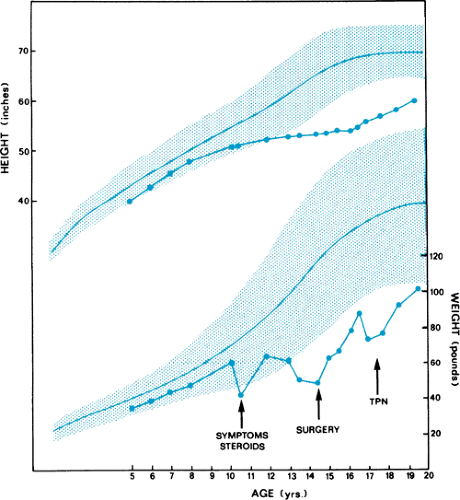
Growth failure may occur with or without corticosteroid therapy. Despite evidence that corticosteroids may suppress linear growth, their use in controlling the inflammation of CD often permits growth to resume at normal rates. Whether accelerated or catch-up growth sufficient to reach the premorbid growth percentiles can be achieved during high-dose corticosteroid treatment is unclear. The patient with long-standing disease may adapt to a state of chronic undernutrition, characterized by height stunted below expected percentiles, appropriate weight for height, and normal to subnormal linear growth velocity. The consequences of untreated chronic undernutrition in a child with CD are poor disease control, increased complications, delayed puberty, and permanent short stature (Fig. 353.2).
COMPLICATIONS
The major intestinal complications of CD are related to the transmural nature of the inflammation that extends from mucosa to serosa. Contiguous loops of bowel or other organs may become enveloped in inflammation. Adhesions, strictures, and abscesses may develop, with a risk of development of an obstruction or bacterial overgrowth. Fistulas may form to any abdominal or pelvic structure and should be suspected to underlie any chronic draining ulcer or sinus. Enterocutaneous, enteroenteric, perirectal, labial, enterovaginal, and enterovesical fistulas may pose a nutritional hazard because they are conduits for major losses of protein and other nutrients. Perianal disease occurs in 25% of patients with CD, most often in the context of colonic inflammation and fistula formation. Skin tags, anal fissures, and perianal or perirectal abscesses may precede other signs of intestinal CD or develop during an exacerbation of colitis. Although often minor in appearance, these lesions can create severe discomfort and be quite refractory to treatment. Massive hemorrhage and toxic megacolon, which are potential complications of UC, occur only rarely in CD.
The risk of malignancy of all types appears to be increased in patients with CD and is estimated to be 20 times greater than normal for patients with CD diagnosed before the patient is 21 years of age. Nonetheless, the incidence of small bowel
carcinoma is low, and the rates of colonic adenocarcinoma are lower than those in patients with UC. However, rates of colorectal cancer approach those of UC when adjusted for the extent of colonic involvement in Crohn colitis. The association of colonic mucosal dysplasia with carcinoma in CD is not established sufficiently to recommend prophylactic colectomy, although performing surveillance colonoscopy and biopsies as in UC is prudent in patients with long-standing Crohn colitis.
carcinoma is low, and the rates of colonic adenocarcinoma are lower than those in patients with UC. However, rates of colorectal cancer approach those of UC when adjusted for the extent of colonic involvement in Crohn colitis. The association of colonic mucosal dysplasia with carcinoma in CD is not established sufficiently to recommend prophylactic colectomy, although performing surveillance colonoscopy and biopsies as in UC is prudent in patients with long-standing Crohn colitis.
DIAGNOSIS
The diagnosis of CD is based on clinical presentation, radiologic findings, and mucosal appearance and histology after exclusion of alternative causes. A complete history should be obtained, with attention given to family history, exposure to infectious agents or antibiotic treatment, extraintestinal manifestations, and retardation in growth rate or in sexual development. Physical examination should include assessment of hydration, nutritional status, signs of peritoneal inflammation, and signs of systemic chronic disease. Features suggesting CD are stomatitis; perianal skin tags, fissures, fistulas, or inflammation; and digital clubbing. Fever, orthostasis, tachycardia, and abdominal tenderness, distention, or mass should be considered indications for admission to the hospital. Disease activity indices have been validated to provide an objective scale for evaluating and measuring the severity or intensity of disease in pediatric patients with CD (Table 353.1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree