Fig. 2.1
Processing pipeline. (a) Original CT image. (b) Segmentation mask. (c) 3D reconstruction of the tibiae and the tumour. (d) Cutting out the tumour in a virtual environment. (e) Illustration of the similarity between the diseased bone and the mirrored version of the contralateral tibia. (f) Illustration of how the template matching algorithm searches through the virtual bone database. (g) Illustration of the good fit of a part cut out from the best matching tibia and placed at the location of the resected section (With kind permission from Springer Science and Business Media)
2.
Virtually cutting out a part of the healthy contralateral bone that corresponds to the location of the tumour (Fig. 2.1d)
3.
Automatic registration of the template with all bones in the databank and storing measured distance metrics (Fig. 2.1f)
5.
Using the boundaries of the registered template to outline the physical cutting planes on the selected bone and extract the allograft
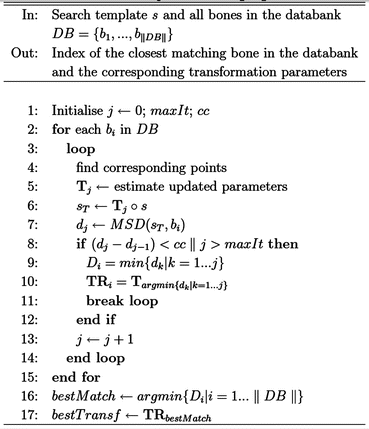
As mentioned earlier, the original anatomy of the diseased bone is extracted from the patient’s healthy contralateral limb and used as a template to guide the search within the databank of cadaver bones. The search is illustrated in the form of a pseudo-code in the algorithm (Please refer to algorithm in the figure below). For each cadaver bone in the databank (line 2), the algorithm applies an iterative closest point- (ICP) based registration on the point clouds of the template and the bone itself to find the transformation that minimises the difference between the two surfaces (lines 4–7). This is done in an iterative fashion and only stops when a certain convergence criterion (cc = 0:001 mm, line 8) is met, or when the number of iterations exceeds a preset value (maxIt = 200, line 8). Surface distance metrics are measured and stored for further processing (line 7). The rigid transformation is then applied to the template to place it in the best fitting location and orientation. This process is repeated until all bones in the databank are examined.
At this stage, each bone in the databank is represented by the minimum surface distance metric between the bone itself and the best fit of the template. Since the goal is to find the closest global match, one or more closely matching donors can be selected (lines 16, 17), thus giving the surgeon one-to-few possibilities to choose from.

Following the initial presentation of the method, a thorough evaluation was carried out where all three methods, namely, the manual selection, the volume-based selection, and the surface-based automatic selection were compared (Bousleiman et al. [16]). All three methods were applied on the exact same data set of the hemipelvis where realistic clinical scenarios of intercalary implants were mimicked.
Results
The automatic allograft selection method based on surface registration showed promising results in its initial presentation. However, its advantages were highlighted when it was compared to other gold standard methods.
Overall, the allografts selected by the surface-based registration method were superior to those selected by other approaches in terms of surface-to-surface fit with the target shape. More importantly, the junctions between the host bone and the intercalary allografts were reported to be significantly better. Additionally processing time was several orders of magnitude lower that those recorded for the manual and the volume registration methods.
Conclusion
Computer-assisted or computer-aided surgery and surgical systems are key components of many modern clinical interventions. Better surgical outcome, shorter surgical time, and improved decision making are typical benefits of using such systems. Development of surgical software and associated tools is a rather popular field among research scientists and industrial manufacturers alike. The field itself has a wide coverage as well. It could include the pre-operative planning, the intra-operative assistance, and the computationally-based design of instruments and tools.
Bone grafting is a common procedure, and several reports supported the use of grafts extracted from cadavers. The selection and planning process is a tedious and time-consuming manual task. In this chapter, a fully automatic method was presented that indicates which cadaveric bone is suitable for a certain patient, and defines how the donor and recipient bones are to be cut. The presented method was thoroughly evaluated and compared to state-of-the-art methods through an active multi-national collaboration with developers of other approaches for the same application. The methodology presented in this chapter outperformed its competing counterparts. The assessment of the method yielded conclusive results that it is at a ripe stage where it can be transferred to a clinical setup for evaluation and long-term patient follow-up.
A particular focus in the evaluation of the method was set on the contact areas between the host and the graft. The quality of surface overlap at the junctions between the donor and recipient is a major aspect that dictates the outcome of the surgery and the difficulty of the transplantation procedure. Furthermore, a smoother transition between the bones facilitates the placement of reconstruction plates and might have positive impact on the incorporation of the allograft into the host bed, thus might decrease the non-union rate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








