(1)
Department of Orthopedic Surgery ASAN Medical Center, University of Ulsan College of Medicine, Seoul, Korea, Republic of South Korea
Abstract
Since the purpose of arthroplasty is to reduce the pain and to maximize function for long term, the surgery cannot be considered successful if these goals are not achieved.
Since the purpose of arthroplasty is to reduce the pain and to maximize function for long term, the surgery cannot be considered successful if these goals are not achieved.
Although it is not clear as to what should be included under complications, it is a generally accepted concept to include all undesirable postoperative conditions under the heading of complications. These comprise of systemic complications and complications pertaining to the knee joint and may be caused due to the factors related to the patient, prosthesis, and/or the surgery.
8.1 Systemic Complications
Systemic complications are more common after TKA than after THA. These include myocardial infarction, pulmonary embolism, shock, cerebrovascular accident, renal failure, urinary retention, ileus, other organ infection, and deep vein thrombosis (DVT). Although not always possible, prevention of these complications is of utmost importance as they can lead to fatal results.
Once complications such as cerebrovascular accident, cardiopulmonary disease, or urinary retention occur, they are beyond the scope of management by an orthopedic surgeon. Although the help of a physician is required for management of complications such as deep vein thrombosis and fat embolism, I would like to describe them here since the orthopedic surgeons are required to diagnose and prevent these complications during the early postoperative period.
8.1.1 Deep Vein Thrombosis
Thrombosis occurs in the veins of the operated limb, and it interferes with the blood flow. When the thrombus migrates into the cardiopulmonary system and causes a pulmonary embolism (PE), fatal results can occur. Sometimes the term VTE (venous thromboembolism) is often used pertaining to both DVT and PE.
8.1.1.1 Etiology
The first stimulus that causes the formation of a thrombus develops during the perioperative period. It is assumed that it is caused by changes in coagulation factors due to the operation, vascular injury, use of tourniquet, and venous stasis due to long-term immobilization of the lower limbs. The use of tourniquet is controversial; Harvey et al. reported that the incidence of thrombosis was not related to the time of tourniquet inflation, but Aglietti et al. stated that the incidence of thrombosis increased when a tourniquet was not used.
8.1.1.2 Incidence
The incidence of thrombosis increases by about 20 times after a major operation. The incidence varies according to patients’ risk factors, method of operation and anesthesia, ambulation period, and preventive anticoagulation therapy.
It is generally known that thrombosis is more common among patients who are older, obese, have cancer, have a history of cardiopulmonary disease, have inherited coagulation disorder, have uncontrolled diabetes, or have been immobilized for long time. Preoperative walking helps in the prevention of thrombosis; Pearse et al. reported that the incidence of thrombosis drops significantly when the patient is instructed to walk 24 h prior to the operation. The incidence of thrombosis after arthroplasty seems to vary among different races.
The incidence of thrombosis is known to decrease when continuous epidural anesthesia and regional anesthesia are used. Some authors reported an incidence of thrombosis of 58 % after simultaneous operation and of 75 % after two-stage operation when preventive measures were not taken. However, these incidences also vary according to the type of thrombosis. Thrombosis can be classified into asymptomatic thrombosis which has no definite symptoms and gradually disappears, and symptomatic thrombosis. The above incidences are those including asymptomatic thrombosis. The incidence of symptomatic thrombosis is 15–25 % and 0.1–2.0 % among them causes pulmonary embolism that can lead to death. Lotke and Lonner reported that the incidence of fatal pulmonary embolism is very low and the correlation between pulmonary embolism and deep vein thrombosis is inconsistent.
The incidence of thrombosis after THA and after TKA is also different. Overall, thrombosis is less frequent after TKA than after THA. Symptomatic proximal vein thrombosis is common after THA. On the other hand, isolated proximal vein thrombosis rarely occurs after TKA, and the majority of thrombotic episodes are due to asymptomatic calf vein thrombosis. Also, 10–15 % of patients experience thrombosis in the unoperated leg.
The time of onset of thrombosis after THA and after TKA is different. The majority of thrombotic episodes occur during the first week after TKA, whereas the majority of thrombotic episodes occur during the first 3–4 weeks after THA.
8.1.1.3 Symptoms
Since most episodes of thrombosis are clinically silent, the only presentation could be in the form of unexpected death.
Post-thrombotic syndrome comprises of three or more of the following symptoms: swelling, skin induration, hyperpigmentation, venous ectasia, redness, pain on calf compression, and venous ulceration. The etiology of post-thrombotic syndrome is venous hypertension caused due to venous occlusion. The symptoms develop due to increased tissue pressure. However, many of these symptoms can occur postoperatively without the presence of thrombosis, so these cannot be considered as the pathognomonic symptoms of thrombosis. Some patients complain of persistent swelling over the calf at the regular follow-up, and I think the diagnosis in such cases would be post-thrombotic syndrome as a result of the development of microthrombi.
8.1.1.4 Diagnosis
Since many cases of thrombosis are asymptomatic, the diagnosis cannot be made based on the symptoms alone. The D-dimer level, duplex sonography, and venography are the common diagnostic tools for DVT, and these procedures should be performed when DVT is suspected.
Since checking the D-dimer level is simple, it is used as a screening test. Fibrin, the most important factor for coagulation, is broken down into fibrin degradation product (FDP) and D-dimer by plasmin, which is a fibrinolytic enzyme. Therefore, an increase in the D-dimer level indicates an increase of fibrin, which means that thrombus formation has occurred. However, the D-dimer level may be increased in inflammatory diseases and cancer. For the interpretation of the D-dimer level, if the level of D-dimer is normal, it suggests that there is no probability of DVT. However, if the D-dimer level is high, it does not always indicate DVT.
Duplex sonography is a good diagnostic method as it is noninvasive, relatively easy to perform and can detect the site and size of the thrombus along with the hemodynamic information of the thrombus. Brassard et al. stated that duplex sonography is very accurate when performed by an experienced examiner. However, it has low specificity and is used as a screening test.
Venography is accurate, but it is an invasive method and should be performed when there is any suspicion of thrombosis on the duplex sonography. In addition, magnetic resonance venography can be performed to confirm the presence of pelvic vein thrombus, and lung perfusion scan or pulmonary angiogram can be performed when pulmonary embolism is suspected.
8.1.1.5 Prevention
Prevention of thrombosis is more important than its treatment not only because it causes fatal complications, but it also leads to chronic pulmonary hypertension due to congestion, ulcers in the lower limbs due to swelling, recurrent thrombosis, and/or chronic venous stasis.
Thrombosis cannot be prevented in all cases with the preventive measures, but its incidence can be decreased according to the method of prevention. Wang et al. emphasized the use of antithrombotic agents in Asian patients, as thrombosis occurred in 71 % of patients who did not take antithrombotic agents and in 45 % of patients who took antithrombotic agents.
It is a general belief that antithrombotic agents cause problems related to bleeding, infection, and wound care. Therefore, both the benefits and drawbacks of using antithrombotic agents should be considered.
There are two basic treatment modalities: One is according to the recommendation of the American College of Chest Physicians (ACCP) and the other is according to the recommendation of the American Academy of Orthopedic Surgeons (AAOS). The basic differences between these two methods are whether to use an antithrombotic agent routinely and whether or not to use aspirin and mechanical devices for DVT prophylaxis.
The classic ACCP guideline recommended the routine use of antithrombotic agents whose effect has been established (excluding aspirin) in the patients who have undergone major orthopedic surgery. They recommended mechanical methods in only bleeding risk group. The ACCP considered all the patients who have undergone major orthopedic surgery as the risk group, and they disagreed on the effect of aspirin as an antithrombotic agent. Recently ACCP group agreed that orthopedic surgery is not as risky as before and modified the guidelines at ninth edition. In the current ninth edition, they still recommended routine use of antithrombotic agents in patients who have received major orthopedic surgery. Among the antithrombotic agents, low molecular weight heparin is more recommended. They recommend the use of IPCD (intermittent pneumatic compression device) in patients in whom bleeding risk is high and adding the IPCD to the pharmaceutical method during hospital stay.
The American Academy of Orthopedic Surgeons (AAOS) objected to the method suggested by the ACCP for the prevention of DVT based on the followings:
1.
The method of arthroplasty that is being used now is different from that used 15 years ago in terms of techniques and operation time. This means that TKA is not a highly risky operation anymore. Hence, the method of prevention should not be based on the old concept of arthroplasty.
2.
Cases reported in the literature after 1996 show that the incidence of symptomatic pulmonary embolism did not decrease with the use of low molecular weight heparin, warfarin, aspirin, or mechanical method.
3.
There is not enough evidence to support the effectiveness of the ACCP recommendation, as the incidence of symptomatic or fatal embolism after arthroplasty is very low.
4.
The risk of bleeding should be taken into consideration.
Novicoff et al. reported that the incidence of DVT did not drop and bleeding and hematoma increased significantly with the use of strict preventive measures as suggested by ACCP.
In fact, the use of antithrombotic agents can reduce the incidence of asymptomatic thrombosis, but there is not enough evidence to support that this type of thrombosis progresses to symptomatic thrombosis. In some cases, preventive methods are used only for legal defense. Therefore, the AAOS largely categorized patients according to the risk of symptomatic pulmonary embolism (HD: high-risk DVT, SD: standard risk DVT) and the risk of bleeding (HB: high-risk bleeding, SB: standard risk bleeding). The patients were divided into four groups: HD and HB, HD and SB, SD and HB, and SD and SB. They suggested more reasonable mitigation guidelines than the ACCP guidelines according to patient grouping and added aspirin and mechanical intervention method targeting INR (International Normalized Ratio) below 2.0. AAOA also modified the guideline recently by changing the DVT risk and bleeding risk criteria. The AAOA limits the high-risk group for DVT only to those patients who have a history of previous VTE and high-risk group for bleeding to those patients who have hepatic dysfunction or coagulation defect.
There are two methods of prevention: pharmaceutical and mechanical intervention.
Pharmaceutical agents should be effective, inexpensive, safe, and little, or no monitoring should be required. Most frequently used drugs include warfarin (Coumadin), low molecular weight heparin (LMWHs), other factor Xa inhibitors such as rivaroxaban and fondaparinux, ximelagatran and aspirin, etc.
Warfarin is the antagonist of vitamin K, and it is available for oral administration and can be used for about 3 months. Khatod et al. reported that only warfarin fared better than mechanical prophylaxis alone, whereas other forms of chemical prophylaxis revealed no significant differences. Disadvantage of warfarin, however, is the narrow therapeutic window; a little less dosage would not be effective and a little more dosage would increase the bleeding tendency. Another disadvantage is that the response to the drug varies significantly from patient to patient, and continuous monitoring is required for the drug interactions. Shorr et al. reported that using warfarin with NSAIDs increased the incidence of hemorrhagic peptic ulcer by 13 times. Also, warfarin takes a longer time to become effective (72–96 h), and it is not as effective in patients of knee arthroplasty as it is in patients of hip arthroplasty.
On the other hand, low molecular weight heparin (LMWH) inhibits factor Xa by activation of antithrombin to prevent thrombosis. Its molecular weight is 1,000–10,000 Da and it has a higher bioavailability and longer half-life than the regular heparin as it does not couple with plasma proteins and it does not attach to the internal walls of the vessels to a great extent. Although LMWH has a drawback that a subcutaneous (SC) injection is required, it is much more effective than warfarin. The risk of bleeding is decreased when it is used for preventive purpose since it does not increase the thromboplastin time. Also, it is relatively safe and monitoring is not required. Nevertheless, there is a higher risk of bleeding with LMWH as compared to warfarin.
Rivaroxaban and fondaparinux are the other factor Xa inhibitors. Rivaroxaban works directly on factor Xa to inhibit thrombin production and can be given orally without monitoring as it is highly specific and stable. However, it increases the risk of bleeding when given with aspirin, nonsteroid analgesics, or anti-inflammatory drugs. This agent should not be used in patients with severe hepatic and renal dysfunction. Jameson et al. compare the results of effects and side effects between LMWH and rivaroxaban and reported that the rivaroxaban had a higher wound complication rate and a lower DVT rate; however, there was no difference in the incidence of symptomatic PE or all cause of mortality. Fondaparinux is an indirect factor Xa inhibitor that becomes effective by bonding with antithrombin. It should not be given within postoperative 6 h as it can increase the risk of bleeding, and monitoring is needed. It is contraindicated in patients older than 75 years of age, weighing less than 50 kg, or who have poor renal function.
There is also a new drug called ximelagatran. It becomes active when it binds with thrombin and also works directly upon the thrombus. It is effective and relatively safe, and monitoring is not required. Colwell et al. reported that ximelagatran is more effective than warfarin in preventing thrombosis without any significant difference in bleeding tendency.
Aspirin inhibits platelet aggregation, thereby preventing thrombosis. It has an advantage of oral administration and low bleeding tendency, and it is cost effective. However, there are different opinions regarding its effectiveness in prevention of thrombosis.
There are controversies regarding the duration of VTE prophylaxis in TKA. Some surgeons recommend the use of antithrombotic agents for about 7–10 days postoperatively as thrombosis mostly occurs between postoperative days 7 and 10 after TKA, while others recommend its use for 4–5 weeks in patients who have a high risk of thrombosis.
Mechanical interventions include graduated compression stockings (GCSs), intermittent pneumatic compression (IPC), and venous foot pumps (VFPs). These methods promote the circulation of venous blood to prevent thrombosis.
The GCSs, in particular, are not very effective in TKA as they are in THA, and this is known to be related to the use of tourniquet. The IPC is effective in patients in whom preventive drugs are contraindicated. It should be applied for 17–20 h a day. The benefit of this method is that there is no risk of bleeding. The veins in the sole of the foot quickly fill and empty the venous blood during walking, and VFPs mimic the effect of walking on the foot. These mechanical interventions can be more effective when used along with the pharmaceutical methods.
Clinically, the methods of prevention can be used either according to the AAOS or AACP guidelines. On the other hand, patients can be simply divided into low-risk, moderate-risk, high-risk, and extremely high-risk groups, and prophylactic method is chosen according to the level of risk. Early gait training can be recommended in low-risk patients, pharmaceutical therapy with gradual addition of mechanical therapy in moderate- and high-risk patients, and heparinization with all other measures in extremely high-risk patients.
Author’s Opinion

Patients who undergo arthroplasty are elderly, and hence, the risk of thrombosis is inevitably increased.
Although the incidence of thrombosis seems to vary among different races, prevention is necessary in order to ensure safety of the patients, to reduce the regional complications of thrombosis, and to protect the surgeon from legal issues.
As LMWHs and other factor Xa inhibitors are relatively safer drugs, they can be used for about 7–10 days after TKA, and the use of warfarin can be considered in high-risk patients even after discharge.
Among the mechanical methods, I do not recommend the use of GCSs as it is less effective. I prefer to use the IPC as it has no side effects and ensures a certain level of legal protection, and patients like to use it as it gives the feeling of a massage. Air cuff is fitted routinely from the operation room after wound closure and is kept for about a week.
Since I have used the IPC routinely, I have observed that the number of patients who complained of calf swelling and pain during hospital stay, and those who have post-thrombotic syndrome on outpatient care, was significantly decreased. I suppose that the IPC prevents the formation of microthrombi.
In high-risk patients, I recommend the use of a combination of pharmaceutical therapy and mechanical methods.
8.1.1.6 Treatment
Once thrombosis develops, it is important to prevent the formation of more thrombi, prevent pulmonary embolism, and treat the pulmonary embolism. It is generally accepted concept that thrombi in the smaller veins can be ignored, but they can propagate in 5–23 % of cases, and 1.6 % of them can cause the symptomatic pulmonary embolism.
When a thrombus is detected, thrombolytics such as heparin, warfarin, and low molecular weight dextran can be used considering the location of the thrombus, patient’s age, and general condition.
The protocol for patients with documented thromboembolic disease by positive venography or ultrasound is that patients with calf, popliteal or femoral thrombi, and asymptomatic pulmonary emboli should be treated with warfarin for 6 weeks. Symptomatic proximal vein thrombi and symptomatic pulmonary emboli should be treated with heparin until the effect of warfarin is established. Physicians recommend the use of heparin injection through the IV route once thrombosis develops, but some orthopedic surgeons recommend the use of heparin injection through the IV route only in the extremely high-risk patient group. Kim et al. reported that it is acceptable not to give any drug unless symptomatic pulmonary embolism develops. The dosage of heparin is determined by the patient’s general condition, and 5,000 U are given every 8–12 h for 1 week to 10 days through IV or IM injection. The problem is that heparin increases the amount of postoperative bleeding. Therefore, the PT (prothrombin time) should be between 15 and 17 s and the PT INR (International Normalized Ratio) should be maintained around 2.0. Careful monitoring throughout the injection period is required as it increases the bleeding tendency if the PT exceeds 20 s or the PTT (partial thromboplastin time) exceeds 50 s. To reduce the bleeding complication, direct infusion of thrombolytic agent into the thrombotic area through a catheter can be performed. But this method is associated with the risk of venous injury.
When pulmonary embolism occurs despite therapeutic warfarin, when warfarin is contraindicated in a high-risk patient, or when complications develop as a result of anticoagulation, Mesh (Greenfield filter) is used as a radiological intervention to prevent the thrombus from propagating into the cardiopulmonary circulatory system. Thrombectomy can be considered. However, this method is indicated in young patients in whom antithrombotic agents cannot be used, since this method can cause vascular injury.
8.1.2 Fat Embolism Syndrome
Fat embolism syndrome is caused due to an increase in the intramedullary pressure while using the intramedullary guide for osteotomy or while inserting the prosthesis with an extension stem.
The incidence of fat embolism syndrome is greater in the one-stage simultaneous operations, when intramedullary guide is used in femur and tibia concomitantly, when excessive reaming is done or the guide rod is inserted vigorously.
Fat emboli have a two-way adverse effect on the body. First, they block the blood supply, thereby burdening the heart (mechanical effect), which occurs mostly in the early stage. Second is the chemical effect that occurs after 48–72 h. The fat is degraded into free fatty acids by lipase, and the free fatty acid increases the capillary permeability and damages the alveolar tissues.
Symptoms include tachycardia, increased secretion, anxiety, drowsiness, and even unconsciousness. The condition may often pass unrecognized as a transient state of confusion after surgery.
There is no definite clinical diagnostic test, but hypoxia in the blood gas analysis and thrombocytopenia are generally observed.
For prevention of fat embolism syndrome, overdrilling of the femoral canal, gently placing the guide rod, copious irrigation, and the use of fluted guide rod are recommended.
Symptomatic management is done, and steroids can also be used when fat embolism has already developed.
8.2 Knee Joint Complications
8.2.1 Pain
Since pain relief is the main purpose of arthroplasty, the operation is considered unsuccessful if the patient suffers from postoperative pain. Pain is closely related to the time elapsed after the operation and is noted in almost all patients during the early stage. However, it can be problematic if the pain is extremely severe even during the early stage, persists for a long time after the operation, or aggravates in comparison to preoperative state.
8.2.1.1 Incidence
When Callahan et al. performed an analysis of 130 studies (9,879 patients) in 1994, 89.3 % of patients reported good or excellent results, 10.7 % of patients reported fair or poor results, and 3.7 % of patients eventually underwent revision arthroplasty at postoperative 4.1 years. With respect to the pain, 75 % of patients reported complete disappearance of pain, 20 % of patients reported mild pain, 3.7 % of patients reported moderate pain, and 1.3 % of patients reported severe pain. Cho et al. reported that 7 % of patients complained of pain to a greater or lesser extent at postoperative 1 year. Elson and Brenkel reported a higher incidence of pain in the first knee of the two-stage operation, in patients who were younger than 60 years of age and who underwent lateral retinacular release, and in whom the posterior cruciate ligament had been sacrificed.
8.2.1.2 Etiology
The causes of pain are largely classified into the intrinsic and extrinsic factors and unexplainable pain. Intrinsic factors are caused due to the problems within the knee joint, and they are further classified into inflammatory factors and mechanical factors. Extrinsic factors are caused due to the problems outside of the knee joint. They are mostly related to the disorders of the spine or the hip joints and are also related to the psychological factors. In some cases, however, the cause of pain is hard to identify clinically.
Intrinsic
Inflammatory
Bacterial infection and other inflammatory diseases or inflammatory reactions cause pain. In such cases, the patients often complain of pain even when they are at rest. Inflam-matory disease is further subdivided into operation related and non-related. Operation non-related category includes rheumatoid arthritis, pigmented villonodular synovitis (PVNS), or Paget’s disease. Inflammatory reactions are associated with joint swelling and pain. If there is fibrosis of the joint, it can limit the joint motion and can cause pain. Patients with allergies to metals also have similar symptoms as those in patients suffering from infection.
Mechanical
Mechanical causes of pain occur due to repeated stimulation of the knee joint, and excessive load or overuse of the knee joint. This type of pain usually increases with activity. The incidence of mechanical pain can vary according to the surgical procedures such as the method of fixation or patellar resurfacing etc. The other causes of pain would be malalignment, instability, limited ROM, loosening and wear, and problems with the extensor mechanism, which are mostly due to surgical failure. Pes bursitis is also a type of inflammatory reaction caused due to irritation of the pes anserinus tendon by the prosthesis
Extrinsic
Extrinsic pain can be divided into operation related and non-related.
Some patients experience pain due to reflex sympathetic dystrophy. Reflex sympathetic dystrophy is one of the complex regional pain syndromes (CRPS). The possible etiology is conduction pathology of the sympathetic nerve due to trauma or surgery. Patients show hypersensitivity to pain even with minimal stimulation. The skin may be cold, discolored, and clammy due to sweating. Saphenous neuroma provokes pain by stimulus.
If the nature of pain is not very different from that before the operation, the cause of the pain is likely to be due to an extrinsic factor which is not related with operation. The patient may complain of persistent pain even though the operation has been successful in terms of alignment, ROM, and stability. In such a case, it is difficult to classify it as a complication of TKA. But persistent or recurring pain makes the patient feel that the operation was not successful. The evaluation of clinical results will show that the knee function score is good, and yet the HSS score is poor.
Elderly patients also have back pain due to spinal stenosis caused by degenerative spondylitis, and this is one of the most common operation non-related factors for knee joint pain. In particular, leg raising exercises after TKA can aggravate the back pain. In most of the patients, radiating pain is accompanied by back pain, while some patients have only mild or no back pain. Since the obturator nerve and the femoral nerve innervate both the hip and knee joints, there can be referred pain in the knee joint when there is a lesion in the hip joint.
Very high expectations, depression, other psychological problems, or secondary gain of the patients may also cause pain. Lundblad et al. reported that the patients with severe preoperative pain are sensitive to the pain sensation and experience more postoperative pain due to low pain threshold.
Mandalia et al. suggested the causes of pain according to the extrinsic and intrinsic factors. The table below is author’s rearrangement of causes of pain based on Mandalia method (Table 8.1).
Table 8.1
Causes of pain according to author’s rearrangement based on Mandalia et al. I. Intrinsic factors in the differential diagnosis of painful total knee replacement. II. Extrinsic factors in the differential diagnosis of painful total knee arthritis
I. Intrinsic factors | |
|---|---|
Operation non-related | Operation related |
I Rheumatoid arthritis | I Infection |
II Pigmented villonodular synovitis | II Instability |
III Paget’s disease | III Malalignment |
IV Overuse | IV Soft tissue impingement Fabellar impingement Popliteus impingement Component overhang |
V Arthrofibrosis | |
VI Wear, osteolysis, and aseptic loosening | |
VII Recurrent hemarthrosis | |
VIII Pes anserinus bursitis | |
IX Extensor mechanism problems Patellar maltracking Patellar clunk Extensor mechanism disruption (patellar fracture, patellar tendon rupture, quadriceps tendon rupture) Unresurfaced patella Undersized patellar button with lateral facet impingement Oversized patellar button with overstuffing Patella baja or alta | |
X Metal allergy | |
II. Extrinsic factors | |
|---|---|
Operation non-related | Operation related |
I Hip pathology | |
II Foot and ankle pathology | I Neurological Neuroma Complex regional pain syndrome |
III Spinal disorders—stenosis/prolapsed disc | II Tendinopathy (patellar/quadriceps) |
IV Vascular | |
V Psychological condition | II Vascular—deep vein thrombosis |
III Heterotopic ossification | |
IV Stress fracture and periprosthetic fracture | |
III. Unexplainable | |
Unexplainable Pain
Some patients continue to complain of pain without any apparent causes for it. Khakharia et al. estimated the incidence of such cases to be approximately 1 in 300 arthroplasties. They presumed that unexplainable pain is likely to occur in patients in whom the preoperative symptoms were worse than the pathologic condition of the knee joint.
Infection, metal allergy, and reflex sympathetic dystrophy should be ruled out first. Overgrowth of the soft tissue, interposed meniscal fragment, lateral facet syndrome, irritation due to a retained osteophyte, extruded bone cement, popliteus tendon dysfunction, collateral ligament irritation caused by medial tibial displacement of the prosthesis, and even hypertrophic pulmonary osteoarthropathy can be the causes of unexplainable pain.
8.2.1.3 Diagnosis
The most important point in the diagnosis of pain is history taking. First, the nature of pain should be identified, followed by physical examination and the necessary tests. Infection should be ruled out first, and then the intrinsic factors should be studied. Extrinsic factors should be searched if no definite intrinsic factors are found. But, too much focus on the extrinsic factors may lead to missing an opportunity for making a timely diagnosis.
History Taking
The history relating to the intensity, site, timing, and provocating and relieving factors of pain should be taken. The first step in making a diagnosis is to identify where and when the pain occurs. The causes for pain are generally intrinsic when the pain is confined to the knee joint.
Neuroma or bursitis is associated with local pain and tenderness in the specific area, and it can be easily diagnosed by history taking. Reflex sympathetic dystrophy may be associated with severe pain and hypersensitivity of the skin in the operated limb. Rheumatoid arthritis may be suspected if the patient has polyarthralgia. If the pain is radiating to the lower limbs, focus should be on the extrinsic origin of pain.
The timing of the development of pain is also very indicative of its probable cause. Inflammation should be suspected if there is pain at rest. Persistent and extreme postoperative pain that does not disappear from postoperative day 1 (pain from day 1 onwards) is a strong sign of an infection. If there is instability or impingement of the soft tissues, pain is experienced from the early stage. On the other hand, delayed pain after a certain pain-free period is likely to be caused by inflammatory diseases, loosening or wear due to instability or malalignment, hematogenous infection, or stress fracture.
If the pain is aggravated by activity, it is likely to be of mechanical origin. Pain on weight bearing is mostly caused by instability and loosening. Pain while climbing up and down the stairs occurs when there are problems with the extensor mechanism such as patellar subluxation, patellar clunk syndrome, or impingement of the patella onto the PE. Pain in extension or flexion that limits the motion is caused by a narrow extension or flexion gap, swelling or hematoma, arthrofibrosis, insufficient removal of the spur, and impingement of the soft tissues.
Bizarre or unspecified pain may be of psychological origin.
Physical Examination
Firstly, it is necessary to observe whether the joint is swollen and if there is a heat sensation. If the joint is swollen, it is necessary to differentiate between swelling due to synovial hypertrophy and simple effusion. Simple effusion is probably due to hemarthrosis or overactivity, while synovial hypertrophy is a manifestation of a chronic inflammatory lesion or chronic infection.
The easiest way to identify the site of the lesion is to palpate for tenderness. Tenderness on the medial side of the knee joint can be caused by overhang of the tibial prosthesis. If there is tenderness on the lateral side of the knee joint, there may be impingement of the popliteus tendon or a problem with the fabella regardless of snapping or clicking. Tendinitis or neuroma can be detected easily by the tender points.
Range of motion, muscle strength test, and instability of joint are also essential tests for making the diagnosis. Since malalignment and rotational deformity can be the cause of pain, they need to be examined as well.
The patella should be mobilized so as to check whether this causes pain. If there is pain along the lateral edge of the patella, it may be due to the lateral facet syndrome caused by the small patellar component in relation to the size of patella.
A lot of information can be obtained by observing the patient’s gait.
If no intrinsic factors can be identified, extrinsic factors such as the spinal or hip lesions need to be examined.
Investigation
After physical examination, X-rays should be taken first. Simple X-rays can show the size and position of the prosthesis, its alignment, wear, and loosening along with patellofemoral alignment. If any abnormal findings are noted, it is important to compare them with the previous X-rays to know what has been changed.
If instability is suspected, stress or weight bearing X-ray should be taken to know the degree of joint laxity. If loosening or wear is suspected, an oblique view is needed in addition to the A–P, lateral, and axial images, or fluoroscopy can be used for making a more accurate diagnosis.
The next step is the study for differential diagnosis of the infection. Laboratory tests for ESR and CRP should be done routinely. If the results are abnormal, other chronic inflammatory diseases should be ruled out. If there are signs of inflammatory diseases, joint fluid analysis and isotope test can be performed based on the abnormal results. Bone scan, especially a WBC scan, is useful for making the diagnosis of infection, and it is clinically more significant when the scan is negative than when the scan is positive.
CT scan is useful for checking the rotational alignment of the prosthesis or when there is osteolysis. MRI is used occasionally to identify inflammatory disease or infection, but the metal interferes with imaging and much time is needed to get rid of metallic artifact. Murakami et al. reported that MRI is an effective modality in evaluating rotational alignment of component as well as extend of synovitis.
Reflex sympathetic dystrophy is diagnosed and tested by lumbar sympathetic ganglion block. Injection of local anesthetics is helpful for the diagnosis and treatment of bursitis or neuroma.
If allergy to the metal (metal sensitivity) is suspected, allergen testing is needed.
Arthroscopy can be performed for obtaining the pathological specimen, removal of foreign body, for adhesiolysis, and for treating the soft tissue impingement such as patellar clunk syndrome, but it is not used only for making a diagnosis.
X-ray, CT, or MRI of the spine or electromyogram can be performed for determining the causes of extrinsic pain.
As vascular diseases such as aneurysm, thrombosis, and atherosclerosis can be accompanied by pain, angiography may be needed if a vascular lesion is suspected.
If no causes can be detected after performing all these tests, consulting a neurologist or psychiatrist should be considered.
8.2.1.4 Treatment
The treatment of pain depends on its causes, intensity, and progression. If there is a definite cause of pain, it obviously requires treatment accordingly. However, the first step is to relieve the pain by medications and observe the progress unless there is an infection or rapid progression. Elson et al. stated that telling the patients that the pain will get better helps to reduce the pain.
Pain that is confined to a certain area such as bursitis or enthesopathy can be treated by injecting local anesthetics and steroids. If the cause originates from the synovial membrane, or if the pain is a result of mechanical block due to particles of PE debris or cement, arthroscopy can be performed.
In saphenous neuroma, simple resection of neuroma increases the possibility of a recurrence. Dellon et al. reported that they could reduce the incidence of recurrence of neuroma by intramuscular placement of the nerve ending or partially resecting a branch of the sensory nerve.
The treatment of reflex sympathetic dystrophy starts with administration of NSAIDs and muscle stimulation. Since reflex sympathetic dystrophy is accompanied by osteoporosis, weight bearing is encouraged and bisphosphonates can be administered. Psychiatric consultation may be helpful. If there is no response to the above methods, sympathetic block or sympathectomy may relieve the symptoms.
If infection, instability, wear, or loosening is the cause of pain and the speed of progression is fast, revision should be considered. Revision surgery is likely to fail if it is done without identifying the cause of pain. Mont et al. performed exploratory revision in patients with and without limited ROM and reported that it was successful in 60 % of patients with limited motion. Exploratory revision was successful in only 17 % of the patients who had pain without limited ROM, and the remaining patients complained of more pain. This means that revision surgery does not help in relieving the pain when its cause has not been identified.
8.2.2 Swelling
Swelling in the early postoperative period is considered normal. In patients who have undergone lateral retinacular release, the swelling would persist for a longer time. When the knee has been overused, it may be swollen even though there is no problem with the knee joint. Patients with rheumatoid arthritis may develop swelling as the disease progresses. Abnormal extensor mechanism, problems with joint mechanics, instability, or arthrofibrosis may induce hypertrophy of the synovial tissue to cause persistent swelling. When the PE is worn out, its particles cause synovitis which in turn causes swelling and pain. There have been some reports of recurrent hemarthrosis or swelling without definite etiology. Although there are numerous other causes that can give rise to swelling, the possibility of infection should be considered first. Hence, the first step in making a diagnosis is to rule out infection, and all tests for infection including arthrocentesis should be performed if there is persistent swelling and pain.
8.2.3 Motion Limitation
Motion limitation not only causes pain but also lowers patients’ satisfaction due to functional disturbance. Motion limitation is the most common indication for revision surgery which does not exchange the implant. More flexion motion is better unless there is any problem with the knee mechanics. Normally, one can flex the knee up to 160°, but maximum flexion would probably be around 145° after TKA. Motion limitation can be classified into flexion contracture and limited flexion. Flexion of at least 95° should be achieved in order to climb up and down the stairs without any handicap, and full flexion is required for squatting. So more than 10° of flexion contracture and less than 95° of flexion can be included in motion limitation. Flexion contracture causes more disability than that due to limited flexion, and hence, flexion contracture should be corrected during the operation. However, in many cases, a flexion contracture of less than 10° during the early stage of the operation can be relieved with the passage of time.
8.2.3.1 Cause
After TKA, the ROM is affected by the patient-related factors, surgical techniques, prosthesis design, and postoperative rehabilitation.
Patient-Related Factors
One of the most important predisposing causes of limited ROM is the preoperative ROM. If the preoperative ROM was severely limited, the ROM would not be as much as that after ordinary arthroplasty due to quadriceps shortening and fibrosis of the soft tissues, even though the operation was successful. Also an extension lag may develop due to weakening of muscular strength when the quadriceps tendon or patellar tendon lengthening has been performed. If the preoperative ROM was less than 75°, the ROM may increase postoperatively, but in most of the cases, it is not more than 110°. Montgomery et al. reported that patients with ankylosed knee achieved an average flexion of 93° after TKA, and Naranja et al. reported that the average flexion achieved was only 62°.
It is believed that obesity affects the ROM, but this is because most patients cannot achieve enough flexion due to excessive subcutaneous fatty tissues on the thigh and calf. Otherwise, obesity does not affect the ROM to a great extent.
Other patient-related factors are preoperative diagnosis, body habits, and depressed patients with low threshold for pain. Patients who are prone to keloid formation may also be prone to develop arthrofibrosis.
Surgical Techniques
The range of motion is closely related to the surgical techniques. Most of the times, motion limitation is due to insufficient removal of spurs, flexion and extension gap imbalance, soft tissue impingement, and poor extensor mechanism.
If the spurs on the posterior condyle of the femur are not removed sufficiently, it causes extension or flexion motion limitation. Gap balancing is an important surgical technique for achieving a good ROM. When both the flexion and extension gaps decrease, flexion contracture and flexion limitation occur at the same time. Flexion contracture occurs when the extension gap is narrow and a narrow flexion gap decreases the angle of flexion. Reducing the posterior slope of the tibia narrows the flexion gap and decreases flexion motion. Excessive joint line elevation causes more tension on the soft tissues when the knee joint flexes by more than 90° and causes motion limitation. Also, excessive tension on the posterior cruciate ligament in the CR type can limit flexion and extension motion, and capsular contracture limits extension. Problems with the extensor mechanism are also an important factor that limits motion. Poor patellofemoral alignment causes the patients to avoid bending the knee joint due to anxiety and instability leading to limited motion. Correct component rotation is critical for patellar tracking, and internal rotation of the femoral and tibial components must be avoided. Bédard et al. suggested that internally rotated components compromised motion because of pain, patellar maltracking, a tight medial flexion gap, and limited femoral rollback on a high-conformity lateral tibial prosthesis. When the prosthesis is too large, it causes pain along with limited motion. Nicholls and Dorr stated that abnormal position of the prosthesis also causes limited motion.
Prosthetic Design
Prosthetic design can also affect the range of motion. Comparing the PS type with CR type, a better range of motion is achieved with the PS type than with the CR type, not only because it secures the rollback but also because it widens the flexion gap. Some surgeons emphasize that mobile-bearing prosthesis can increase the range of motion, while many surgeons report that it does not show any significant difference in the ROM. Constrained prosthesis not only interferes with the extension or flexion motion but also decreases the rotation and medial/lateral motion. There are some prostheses which increase the flexion motion, so-called high flexion knee. Many authors report that the use of “high flexion knee” increases the range of flexion motion, while others report no difference between conventional and “high flexion knee.”
Postoperative Care
The ROM is also closely related to postoperative wound healing. If there is an excessive hematoma or wound healing is delayed in the early postoperative period, the pain limits the rehabilitation process and exercise is restricted during the treatment of the wound.
Swelling of the joint stimulates the mechanoreceptors in the joint capsule, thereby limiting the range of motion, and hence, persistent swelling results in limited ROM.
The ROM can be decreased if the patient is too anxious, lacks motivation, or is uncooperative with rehabilitation due to the abnormal response to pain and poorly controlled pain in the early postoperative period.
Miscellaneous
Other causes include infection, arthrofibrosis, and reflex sympathetic dystrophy. Arthrofibrosis, which develops as a result of combination of the aforementioned causes, is one of the common causes of stiffness following TKA. In cases in which the ROM is good at first but becomes progressively limited later, we should suspect any of the following causes: infection, synovitis due to overuse, rheumatoid arthritis, wear debris, recurrent hemarthrosis, loosening or damaged prosthesis, etc.
8.2.3.2 Treatment
Management is not very easy when the ROM is limited. The conservative treatment in the form of physiotherapy should be tried first and followed by graduated treatment protocol. When the desired ROM cannot be achieved, manipulation, brisement under anesthesia, arthroscopic adhesiolysis, open adhesiolysis, and revision TKA can be considered in that order.
Before revision arthroplasty is undertaken, the cause should be identified and the extrinsic factors should be ruled out.
If the range of motion is less than 95° until postoperative 4–6 weeks, more aggressive physiotherapy or manipulation can be considered. When this is done within postoperative 3 months, the results are promising. Numerous authors have reported successful results with manipulation. But manipulation is still a controversial issue. Brassard et al. and Maloney et al. stated that manipulation can be attempted during the early stage of rehabilitation and brisement under anesthesia between postoperative 6 and 12 weeks achieved good results when flexion was less than 75°. However, they advised that the procedure should be performed very carefully as it can cause a fracture or an avulsion fracture. The purpose of manipulation is to reduce intra-articular adhesions in order to normalize the early postoperative rehabilitation process and reduce the risk of developing a permanent limited ROM. Cates and Schmidt evaluated the stiff TKA and found that manipulation was most successful when it was performed within 8 weeks and in patients with full extension and at least 90° of flexion. Witvrouw et al. used computer-controlled motion technology (Antwerp, Belgium) device to provide static progressive stretch utilizing computer-controlled low-lead prolonged force so as to improve ROM. They reported that this device had equal or superior result than manipulation under anesthesia, especially in relieving flexion contracture.
Adhesiolysis using arthroscopy is another option. Adequate timing of arthroscopic arthrolysis is 3–6 months after TKA. Arthroscopy is done through 2–3 portals as in the usual arthroscopic surgery, but the prosthesis should not be damaged so as to avoid the development of scratches and wear particles. The joint is examined first without knee motion and is checked for adhesions. After this, it should be checked whether the soft tissues are impinging into the joint during knee motion. The impinging soft tissues should be removed, and any adhesions should be released using a trocar, basket forceps, or shaver. Diduch et al. demonstrated that adhesiolysis using arthroscopy increases the ROM by an average of 26°. Mont et al. also reported that adhesiolysis using arthroscopy was successful in patients with limited motion without any apparent problem on the X-ray.
In the case of severe ROM limitation with more than 20° of flexion contracture and less than 70° of flexion motion, arthroscopic treatment alone is less effective and open adhesiolysis is needed. It is desirable to perform adhesiolysis within 1 year of primary surgery. If such a limited motion is caused due to patellofemoral malalignment or overstuffing of the extension and flexion gaps, there is no other option besides revision surgery. However, revision surgery is not always successful if there is extensive scar formation due to arthrofibrosis or the causes of limited ROM are unclear. Revision surgery should include adhesiolysis and soft tissue balancing. Nelson et al. reported that revision of soft tissues improved the ROM in patients with an arc of motion between 15° and 75°, but the overall range of motion was improved up to a certain level. Bellemans et al. proposed a treatment method for flexion contracture. They divided the procedure into four steps, and the results were positive. The first step of the algorithm is removing the spur completely and increasing the resection of the distal femur by 2 mm and correcting the medial/lateral balance. The second step is gradually releasing the posterior capsule and gastrocnemius muscles. The third step is resecting the distal femur up to 4 mm, and the last step is performing hamstring tenotomy.
8.2.4 Instability and Dislocation
Instability is the second most common cause of revision surgery following infection, which is performed within 2 years after the index operation. The definition of instability is controversial, but it is accepted that instability is considered to exist when there is more than a 2 mm medial opening and a 3 mm lateral opening in extension and a 3 mm medial opening and a 4 mm lateral opening in flexion. A–P instability is defined if the tibia can be displaced by more than 5 mm or the joint can be dislocated. Also, the patella should not subluxate or disclocate.
Knee instability makes walking difficult and causes joint swelling and pain. When giving way on walking is accompanied with vague anterior knee pain, recurrent effusions, and soft tissue tenderness, instability should be suspected. In severe cases, it may cause joint dislocation. Also, it worsens the prognosis as it causes wear or loosening from the early stage. This is more prominently seen when the prosthesis has a flat articular surface.
8.2.4.1 Cause
Even if the operation was successful, instability occurs if there is not enough muscular strength to support the joint. Therefore, operation cannot be indicated in the patients with neuromuscular disorders. However, neuromuscular disorder may develop postoperatively and can cause instability of the operated joint.
Most of the times, instability is caused due to problems of the knee joint itself. Early instability may be a result of surgical failure including malalignment, imbalance of flexion–extension gap, inadequate correction of the deformity, failure to achieve mediolateral balance, rupture of the PCL in the CR type, etc. Late instability is caused due to overuse of the joint and trauma, or it is secondary to complications such as infection, loosening, or wear.
Instability is classified into anterior instability, instability in extension, instability in flexion, genu recurvatum, and global instability, and the causes and findings in each type of instability are different. In general, instability in extension manifests as mediolateral instability or genu recurvatum, while instability in flexion is manifested as A–P instability.
Anterior instability is associated with quadriceps weakness or subluxation or dislocation of the patella. In such a case, the patient shows quadriceps avoidance gait in which the patient is not willing to bend the knee and tends to lean forward while walking.
Instability in extension is further classified into symmetric instability and asymmetric instability, and both types of instability tend to be worsened by malalignment and masked by good alignment. Symmetric instability is caused due to a wide extension gap. It occurs due to over-resection of the distal femur or by using a thinner PE to correct flexion and extension gap imbalance, and when incompletely released, collateral ligaments get detached during walking. Asymmetric instability is more common, and it is mostly due to inadequate release of the collateral ligaments on the contracted side. In such a case, the contracted side is compressed and the other side is more opened (Fig. 8.1), leading to a seesaw effect. Insall stated that valgus instability from over-release of the medial structures during correction of a fixed varus deformity is rare. Koo and Choi reported that there was no significant difference between the MCL-preserved TKAs and the knees with an iatrogenic MCL injury. It may also occur due to sinking down of the bone on one side when the prosthesis is fixed to the weak bone or in case of valgus or varus tibial osteotomy.

Fig. 8.1
Compression on one side leading to opening on the other side
Symmetric instability in flexion mostly occurs when the flexion gap widens or when the PCL is attenuated in TKA performed with the CR type. It also occurs when the flexion gap is excessively widened due to the use of a femoral prosthesis that is too small, the use of a thinner PE for correcting the flexion contracture, or when the posterior cruciate ligament is sacrificed. If it is severe, it can lead to posterior dislocation of the joint. Asymmetric instability in flexion occurs when soft tissues are released to a greater extent for correcting the varus–valgus deformity, and the rotation of the femoral prosthesis is incorrect.
The joint can be unstable in midflexion which makes it difficult to climb the stairs. This instability happens because balance is mostly tested in full extension or at 90° flexion during the operation. Midflexion instability leads to progressive stretching of secondary restraints, and accelerate PE wear. But it is often masked due to tightness of the posterior capsule in full extension. The joint line elevation affects the ligament tension at an intermediate angle to cause midflexion instability. The shift from a longer radius in extension to a shorter radius in flexion with a multi-radius design has been reported to cause instability between 30 and 45° of knee flexion.
Genu recurvatum can occur when the strength of hamstring muscles weakens or the extension gap is too wide. It can also develop with the use of the CR type of prosthesis when the posterior cruciate ligament gets absorbed in patients with RA or when the recessed posterior cruciate ligament gets ruptured.
Global instability occurs when one or more causes of instability occur in combination and when one-plane instability lasts longer.
Subluxation may occur with the use of the CR type of prosthesis, but subluxation is often overlooked because it reduces spontaneously. Dislocation is the more aggravated form of instability. There are different opinions regarding the mechanism of knee dislocation. It is generally believed that dislocation occurs when a backward force is applied while the knee is slightly bent or when posterior and rotational forces are applied while the knee is in a flexed position. In patients with a valgus deformity, dislocation occurs when their legs are crossed. The incidence of dislocation is higher in patients with a preoperative valgus deformity. Lombardi et al. found that the incidence of dislocation was higher in patients who had more flexion motion.
Dislocation has been experienced more frequently with the use of the old PS type of prosthesis. Dislocation is related to location of the cam and height of the post, which is represented by the dislocation safety factor (DSF). Kocmond et al. defined the dislocation safety factor (DSF) as the vertical distance from the bottom of the femoral cam to the top of the tibial spine. The DSF increases as knee flexion increases, and it peaks at 70° of flexion and decreases thereafter, thereby increasing the risk of dislocation. The early PS type design had a lower post and the post was located on the posterior side, and hence, dislocation developed easily. Currently, improved prosthetic design with an increased jump distance has reduced the incidence of this complication. In a mobile-bearing joint, dislocation of the mobile-bearing prosthesis can occur along with instability when ligament balancing cannot be achieved.
8.2.4.2 Treatment
The method of treatment varies according to the type and degree of instability. If the instability is mild, the patients learn how to walk without difficulty. Conservative treatment is recommended initially. If the patient feels unstable during the first postoperative gait, the instability of the knee joint should be evaluated. If no problem is detected on the stress test, it is because of weak muscular strength, and more enhanced muscle strengthening exercises should be recommended. If muscular strength has been weakened due to a neurologic disorder, it needs to be addressed. If there is asymmetrical instability due to ligament or soft tissue imbalance in early stage, it is necessary to fit a brace for approximately 6 weeks for the soft tissues to become scar bound. If ligament instability is neglected at this time and the patient is allowed to walk without brace fitting, the ligament or soft tissues will heal in a relaxed state, and the instability will persist.
If moderate instability is not corrected by improving muscular strength or with the use of brace fitting, revision surgery is needed as it causes the knee to give way, and frequent subluxation or dislocation will cause early wear and loosening.
In symmetric instability in extension and genu recurvatum, exchange to the thicker PE and using a smaller femoral component is needed. In asymmetric extension instability, the contracted side should be released, and the use of a thicker PE is required. Pagnano et al. reported that replacing the CR type with the PS type of prosthesis showed good results.
Flexion instability can be managed by upsizing the femoral component following femoral posterior augmentation. Vince et al. described that it is important to align the limbs and balance the extension and flexion gaps. They concluded that when a constrained prosthesis is used, good results can be expected even though the collateral ligaments are imbalanced. But in case the discrepancy between the flexion and extension gaps is marked, loosening developed eventually, even though the initial results were good.
Acute dislocation can be reduced by pulling the tibia forward while the knee is fully bent, but revision is desirable according to the aforementioned principles of treatment since the possibility of recurrence of dislocation is high. In case of recurrent dislocation, it is required to perform revision and to insert a larger PE or perform component revision using a constrained type of prosthesis. Some authors reported that arthrodesis was inevitable in severe cases.
8.2.5 Vascular Complications
Direct injury to the vessels during arthroplasty is very rare. Popliteal arteries may be injured and aneurysm, pseudoaneurysm, or A–V fistula may develop during adhesiolysis in revision surgery, during posterior capsular stripping in patients with limited motion, or in limb salvage operation with the use of tumor prosthesis.
Yang et al. reported that the most dangerous zone of vascular injury during the operation is just lateral to the posterior center of the lateral condyle at the joint level, as the neurovascular structure is closest to the operation field at this area. Geertsema et al. experienced three cases of popliteal pseudoaneurysm. The most common mechanism for pseudoaneurysm formation is indirect trauma, which includes mechanical stretching, posterior capsule release, dislocating the knee, and thermal injury. They achieved successful revascularization by endovascular stenting.
Most of the times, the preoperative vascular disease is aggravated after the operation. Absolute vascular contraindications to TKA include the presence of confirmed vascular claudication on minimal or no activity, skin ulceration secondary to arterial insufficiency or venous stasis, and ischemia or necrosis of the toes.
If there is injury to the vessels during the operation or the vessels are occluded due to thrombosis, a vascular surgeon should be consulted emergently for seeking his/her help in treating these problems.
8.2.6 Nerve Palsy
Tibial nerve palsy can occur, but peroneal nerve palsy is the most common after TKA. Recently, some authors reported that tibial nerve palsy occurred in minimally invasive surgery when cement was pushed back to the posterior side.
Peroneal nerve palsy is caused due to excessive stretching of the nerve while correcting a severe valgus deformity or flexion contracture, injury during revision, fascial compression, disruption of vascular supply to the nerves, or external pressure, etc. Among them, external pressure is the most common cause of peroneal nerve palsy. Idusuyi and Morrey demonstrated that valgus deformity is the most common preoperative factor causing nerve palsy, and the most common postoperative factor is incorrect position of the limb, while proprioception is lost with epidural block. Other causes of nerve palsy could be the compression by bone or cement, improper use of tourniquet, or compartment syndrome due to excessive bleeding.
The method of treatment depends on the cause of nerve palsy. Palsy caused by external pressure in the early postoperative stage is temporary, and it gradually recovers with time when the elastic bandage is loosened and the knee is positioned at 30–60° flexion. Early detection of palsy generally leads to a better prognosis. Tourniquet palsy requires observation. But palsy as a result of compartment syndrome needs active management such as surgical decompression.
If nerve palsy persists for more than 3 weeks, electromyogram is necessary to identify the site and extent of nerve damage. It is recommended to fit an ankle foot orthosis and perform passive ankle ROM exercises to prevent ankle contracture.
Recovery starts from the motor function of the nerve, and most of the nerves recover without motor weakness; however, disorders of the sensory function often persist. Recovery may be delayed or impossible if there is excessive stretching of the peroneal nerve during the operation or if there is a complete palsy. Kim et al. reported that eight out of nine patients with peroneal palsy recovered, but one patient did not recover.
Krackow reported good results by performing exploration when palsy persisted for more than 2 months. Zywiel et al. also emphasized that nerve decompression improved the sensation and decreased the pain, and they recommended surgical decompression for patients who failed to improve with conventional conservative treatment. However, most of the surgeons agree that exploration is not recommended in the early stage.
8.2.7 Component Breakage
The causes of component breakage are multiple: design and material defects of the prosthesis, poor surgical techniques, and trauma. Most of the times, component breakage occurs when the hinge type or linked type of the constrained prosthesis is used for the young and active patients.
It is also closely related to the prosthesis design. It occurs when there is a groove or screw hole in the prosthesis; when there is no enhanced peripheral rim, with the use of porous coated prosthesis; or when a very thin cobalt chromium metal is used. The femoral component is broken when the femoral runner is thin; Whiteside et al. reported 32 cases of component breakage at the corner and the junction area out of 16,000 cases treated with Ortholoc II cementless prosthesis. The reduced thickness of the metal in these critical areas predisposed to the development of a fracture. Porous coating of the interface further weakened the prosthesis, and sintering process can degrade the quality of the base metal itself.
Some cases of tibial component breakage near the posterior cruciate ligament insertion site have been reported. Chatterji et al. analyzed 25 cases of tibial component breakage and reported that breakage occurred when the supporting bone showed an osteolytic defect, when the bone graft collapsed, or when avascular necrosis developed. In case of such bone defect, eccentric loading and condylar lift-off due to malalignment or PE wear impose an ongoing load causing a fatigue fracture.
The PS type of prosthesis occasionally shows wear and breakage of the post. The incidence of post fracture is higher in younger, obese, and male patients. It is caused due to the design defect, surgical failure, or both. Implants with a hole in the post for screw fixation, anteriorly placed post, and too closely located post and notch are predisposed to post wear and breakage. Hamai et al. and Akasaki et al. revealed that stress on the post is different in each implant and when stress exceeds the compressive yield strength of the post, it causes post wear and breakage. Surgical errors such as valgus malalignment, wide extension gap, instability, and excessive posterior slope predispose to a post fracture. Among them, post wear and fracture occur most frequently when the post is under too much stress to block hyperextension during extension.
When the prosthesis is broken, it causes instability, pain, or deformity, and revision is required.
8.2.8 Complications of the Extensor Mechanism
Complications of the extensor mechanism are the most common complications following TKA. Complications which are related to the extensor mechanism are anterior knee pain, extensor mechanism rupture, patellar fracture, malalignment or dislocation of the patella, patellar clunk syndrome, avascular necrosis of the patella, wear and loosening, etc.
The range of incidence is very wide from 5 to 60 %, because the scope for the complications differs according to the surgeon. Kim et al. reported an incidence of about 29 %, and subluxation of the patella was the most common complication. Meding et al. reported that patellar fracture and loosening of the patellar component were the most common complications excluding anterior knee pain and the risk of fracture was highest when lateral retinacular release had been performed in patients whose BMI was greater than 30. They also reported that the position of patellar component, thickness of the patella, and preoperative ROM and alignment are closely related to loosening. The incidence of these complications is decreasing with the improvement of the prosthesis and the evolution of surgical techniques, but there are still many unsolved problems. Non-resurfacing of the patella is preferred by some surgeons because of these complications, but non-resurfacing does not guarantee a reduction in the incidence of these complications.
Complications of the extensor mechanism not only cause pain and swelling, but they also alter the joint mechanics to aggravate the wear and loosening of other components and affect the survival of the prosthesis.
8.2.8.1 Anterior Knee Pain
Cause
Anterior knee pain is not always associated with the complications of the extensor mechanism itself, and its cause is often unclear. However, it is mostly related to the problems with the extensor mechanism.
Pain can occur if the stress imposed on the extensor mechanism exceeds the physiologic limit during squatting or climbing up and down the stairs. Also, overstuffing of the anterior knee joint or malalignment increases the diffuse or focal pressure, thereby causing anterior knee pain. Meftah et al. reported that the incidence of anterior knee pain at the 10-year follow-up was 22.2 % and that it was partly related to a specific implant used. Although it is controversial, pain is known to be more common among patients in whom the patella has not been resurfaced; Scott stated that pain is mostly due to the progression of the non-resurfaced lesion. Other causes of anterior knee pain are joint swelling, fibrosis of the anterior fat pad, neuroma, and tendonitis.
Treatment
Treatment varies according to the degree and cause of pain. If the pain is not severe, observation is needed; however, moderate pain requires conservative treatment such as medication or physiotherapy. In some cases, steroid injections can be used. When the pain is severe, the patella can be resurfaced if it is thought that the pain is caused due to the unresurfaced patella. However, only patellar resurfacing may not solve this problem in most of the cases. Hence, revision of all components may sometimes be necessary.
8.2.8.2 Rupture of the Extensor Mechanism
If there is rupture of the extensor mechanism, the extension function of the patella is lost, resulting in a very severe functional disturbance. The rupture of the extensor mechanism includes avulsion of the patellar tendon from the tibial tuberosity, patellar fracture, and quadriceps tendon rupture. Patellar fracture is further described in this chapter under Sect. 8.2.16.
Cause
General medical conditions leading to rupture of the extensor mechanism include rheumatoid arthritis, diabetes mellitus, chronic renal failure, hyperthyroidism, and steroid abuse.
Intraoperative rupture occurs when an excessive force is applied to improve the ROM in the patients with quadriceps contracture, ankylotic knee, osteoporosis, weak patella, or in revision cases.
Rupture may occur postoperatively when too much patellar osteotomy was done, too much fat pad was removed, lateral retinacular ligament was released, and transverse incision over the quadriceps tendon was made too close to the superior pole of the patella during paramedial arthrotomy. Malalignment of the extensor mechanism and severe global instability also provoke extensor tendon rupture. It also occurs when manipulation is applied to increase the ROM in the patients who have undergone a rectus snip or V–Y quadricepsplasty.
Infection and trauma are other causes of rupture of the extensor mechanism.
Diagnosis
Most of the patients complain of loss of extension even by a minor indirect trauma, and they may have no definite pain. Physically, dimpling is noted in the area of rupture. X-ray shows patella alta or patella baja. The site and degree of rupture can be detected on sonography or MRI.
Treatment
In case of partial tendon rupture, conservative treatment can yield good results. In case of complete rupture, repair or reconstruction of the tendon should be performed. Excessive tension and circulatory disturbance interferes with the healing. So, thoughtful planning should be estabilished before operation. In other words, medial incision used in the approach disrupts the vascular supply of the tendon from the medial side and the lateral inferior genicular arteries are often injured during meniscectomy or fat pad removal. If lateral retinacular release has been done, vascular supply is almost completely interrupted and direct repair of the tendon is likely to fail. Wilson and Venters performed primary repair of the patellar tendon rupture, and the operation failed in every case. Therefore, semitendinosus tendon or gracilis tendon autograft can be used for augmentation while keeping the tibial insertion attached, or the tendon should be reconstructed with tendon allograft. Cadambi and Engh performed autograft using semitendinosus tendon, Wilson et al. used plantaris autograft, and Jaureguito et al. used the medial head of the gastrocnemius with satisfying results. Emerson et al. recommended the use of allogeneic tendon graft, and I also obtained good results by performing allograft augmentation. A fresh-frozen allograft is a good candidate as an allogeneic tendon. The Achilles tendon is the most widely used. Zanotti et al. introduced the bone–patellar tendon–bone complex allograft and achieved a good success rate.
The tension is the key point during tendon reconstruction. Emerson et al. noted an average extension lag of 28° in 33 % of patients when the maximum tension was applied with the knee flexed at 60° under anesthesia. However, Nazarian et al. and Burnett and Bourne recommended applying maximum possible tension while the knee is extended. This is because the allograft has a tendency to elongate and the ruptured portion of the tendon is likely to elongate as it is already shortened. Lionberger and Correia recommended reinforcement with a dynamic and flexible leash to limit excursion of the patellar tendon while the repaired tendon heals.
For postoperative care, Nazarian et al. recommended the use of a plaster cast in extension for 6 weeks.
Author’s Method
My method for allograft augmentation in case of patellar tendon rupture is to reinforce the repaired tendon using allogeneic hamstring tendon. First, I drill a hole distal to the tibial tuberosity, and the tendon is passed through this hole. The both ends of the allogeneic tendon are sutured to the soft tissues around the patella (Fig. 8.2). For quadriceps tendon rupture, I use Achilles tendon or iliotibial band to reinforce the repaired portion of quadriceps tendon.
I apply maximum tension during allograft augmentation while the knee is extended. After this, tension band suture technique is used to aid in postoperative rehabilitation.
For postoperative care, I identify the flexion angle that does not disrupt the sutures during the operation and start flexion exercises up to 20° less than this angle and avoid straight leg raising or active extension of the knee joint for the first 4–6 weeks.
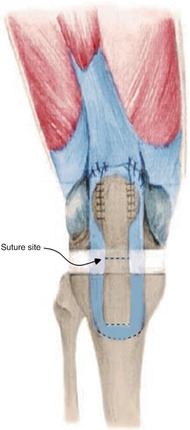

Fig. 8.2
Reconstruction of patellar tendon rupture using allograft. Proximal allograft is sutured to retinacular ligament and quadriceps tendon. Distal allograft is passed through the tibial bone
8.2.8.3 Patellar Subluxation or Dislocation
Maltracking of the patella can cause anterior knee pain, limited ROM, and quadriceps weakness. The rate of wear and loosening and the incidence of patellar fracture can be accelerated due to the changes in the extensor mechanics (Fig. 8.3). However, Rand et al. reported that only 1 % of patellar maltracking is actually associated with clinical symptoms.
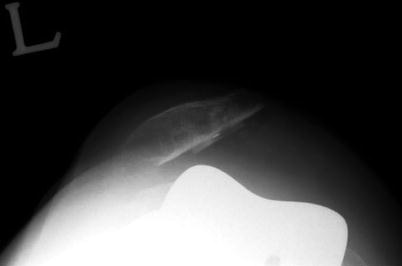

Fig. 8.3
Patellar dislocation
Cause
The causes of patellar subluxation or dislocation are largely divided into patient-related factors, prosthetic designs, and surgical techniques.
Chew et al. reported that there was an average of 5° postoperative patellar tilt even when patellar tracking was good during the operation, and this was closely related to the patient’s preoperative patellar tilt. Preoperative valgus deformity is one of the predisposing factors of subluxation and dislocation. Patients who had severe preoperative knee joint effusion have a distended synovial membrane, and this can be a cause of dynamic patellar instability during extension postoperatively, even though the tracking is good in the operation field.
Prosthetic design is closely related to patellar tracking. Currently, patellar tracking has improved since the patella and the patellar groove have been modified into a patella-friendly shape.
Surgical techniques are the most important factors for good patellar tracking. First, the tibiofemoral alignment should be correct. Valgus deformity, if left uncorrected, causes maltracking. The position and rotation of the prosthesis are also important. Patellar tracking is improved by placing the tibial and femoral prosthesis laterally and the patella medially, and externally rotating the tibial and femoral components. Patellar instability also increases when the patella is resected asymmetrically or the resected patella is too thick. The incidence of subluxation and dislocation also increases when the femoral prosthesis is too large or the lateral retinacular ligament is too tight. An anatomically shaped patella may aggravate maltracking if rotational alignment of the patellar component is not correct. Patellar instability can also occur when the arthrotomy site is disrupted due to excessive swelling, vigorous physiotherapy, or trauma.
Treatment
If there is no pain or if the subluxation is not severe, observation is needed and VMO (vastus medialis obliquus) strengthening exercises should be started.
In case of severe dislocation or if there is accompanying pain, the simplest method that can be performed is lateral retinacular release and medial reinforcement when the prosthesis is in its optimal position. Arthroscopic lateral retinacular release is performed occasionally. The next step, according to Grace and Rand, should be performing a proximal realignment procedure such as VMO advancement or V–Y quadricepsplasty for quadriceps balancing. Whiteside et al. suggested performing tibial tubercle osteotomy, but Rand et al. are not in favor of performing this procedure because it causes many complications such as pain and nonunion. Nakajima et al. reported that Elmslie–Trillat operation and/or extensive lateral retinacular release showed good results.
If the prosthesis is not in its optimal position, lateral retinacular release can be tried first. If the problems are not solved after the lateral retinacular release, revision surgery should be performed to correct malposition and malrotation of the femoral, tibial, and patellar component.
8.2.8.4 Patellar Clunk Syndrome
Patellar clunk syndrome develops when the scar tissues located at the junction of the quadriceps tendon and patella, and causes irritation during flexion and extension motion. Such tissue growth is thought to originate from exposed cancellous bone at the margin of the components or due to chronic irritation of the synovium by the prosthetic prominences. Beight et al. noted that the causes of patellar clunk syndrome originated from the design problems of the femoral prosthesis, postoperative inflammatory reaction, and changes in the extensor mechanism.
Patella clunk syndrome occurs when the knee is actively extended from 45 to 60° of flexion (Fig. 8.4). Catching occurs during the postoperative 6–12 months. This syndrome was more common with older PS type in which the femoral notch portion was extended proximally. The current designs have a longer and deeper femoral sulcus which gradually adjusts to the shape of the femur and helps in reducing the incidence of patellar clunk syndrome significantly.

Fig. 8.4
Photography of patellar clunk syndrome. Fibrous nodule is picked up by forceps
The results of treatment by removing the soft tissues using arthroscopy or open surgery are good. Diduch et al. demonstrated a success rate of 82 % by removal of the fibrous nodules using arthroscopy.
Synovial entrapment or hyperplasia is an entity similar to patellar clunk syndrome, but it is less well described. The location of the lesion is nearly the same as that in patellar clunk syndrome, but there are no discrete nodules. Rather than a clunk or catch, the patient experiences pain and crepitus, typically when the knee is extended actively from 90° of flexion. Treatment of synovial entrapment or hyperplasia depends on the degree of symptoms. If the symptoms (pain and/or crepitus sound) are mild, assurance of the patient is enough. If the symptoms are severe, arthroscopic examination followed by synovectomy is needed.
Patellar crepitus is defined as a palpable, continuous grinding sensation, and the symptoms of patellar crepitus are secondary to those of peripatellar fibrosynovial hyperplasia which develops at the junction of the superior pole of the patella and the distal quadriceps tendon. Dennis et al. reported that the causes of patellar crepitus are multifactorial including previous knee surgeries, decreased component size and composite thickness, shorter patella tendon length, and increased femoral condylar offset. Crepitus which is not related with pain needs observation. If the crepitus is accompanied with pain, the causes of pain should be searched and corrected.
8.2.8.5 Avascular Necrosis of the Patella
The incidence of avascular necrosis of the patella increases when the superior lateral genicular arteries are injured during lateral retinacular release. Avascular necrosis of the patella has the tendency for revascularization in 6 months to 1 year.
It is unclear as to whether or not avascular necrosis of the patella causes anterior knee pain, and there are also various theories regarding its contribution to patellar complications such as loosening and fracture. Theoretically, it is believed that it contributes to the incidence of patellar fracture.
8.2.8.6 Wear and Loosening
When the patellar prosthesis is under excessive load, the incidence of wear and loosening is increased. Meding et al. reported that obesity predisposes to patellar component loosening up to 6.3 times. Loosening can develop due to repeated squatting and when the bone quality is not good due to bone necrosis. The incidence of wear appears to increase with the patient’s weight, postoperative range of motion, and the duration of implantation. The surgical factors that increase the wear are malalignment of the extensor mechanism, joint line elevation, and lateral retinacular release. Vanbiervliet et al. demonstrated via an in vitro study that patellar maltracking due to an internally rotated femoral component may lead to an increased patellar component wear. Prosthetic design also plays an important role; metal-backed patella causes wear more rapidly due to thin PE. The current all-poly patellar component has a significantly reduced incidence of wear.
When wear or loosening develops, the prosthesis should be removed and the condition of the remaining patella should be checked to decide whether or not to re-resurface the patella. In low-demand patients, simple patellar component removal and patelloplasty using an arthroscopic burr may suffice. However, early wear and loosening in young patients might need revision of the femoral or tibial component according to its cause.
8.2.9 Hematoma and Wound Drainage
It is not surprising that a postoperative hematoma can occur as the TKA procedure involves numerous bone cuts, intramedullary canal violation, and soft tissue release.
There are multimodal approaches for minimizing the intraoperative bleeding. Plugging the intramedullary canal, not performing the lateral retinacular release by accurate patellofemoral balancing, and meticulous hemostasis can decrease the initial blood loss. However, to deflate the tourniquet and intraoperative hemostasis is a controversial issue.
The incidence of infection is increased due to prolonged wound drainage. Patel et al. stated that the serous discharge may persist for a long time in patients who are morbidly obese, take anticoagulants, or have excessive drainage.
When a postoperative wound drainage occurs, the first step is to take a wound swab, and a period of immobilization and observation is needed. If the discharge is profuse and if it increases with joint motion, the discharge may be coming from inside of the joint. If the bleeding continues and is suspected to be originated from inside of the joint, the knee can be reopened and the source of bleeding should be identified. Indications for surgical evacuation of a hematoma include wound dehiscence, impending skin necrosis, persistent sanguineous leakage from the incision site, and severe pain. Probing and squeezing the wound to evacuate the hematoma are not recommended because these could lead to retrograde contamination.
8.2.10 Superficial Infection and Skin Necrosis
When wound healing is not satisfactory, there may be a serous discharge from the wound, crust formation, or skin necrosis and superficial infection. When skin necrosis develops, it should be regarded as superficial infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








