Fracture
Anticoagulation/bleeding diatheses
Soft tissue injury
IV fluid extravasation
Dialysis/nephrotic syndrome
Burns
Snake bites
Revascularization
Exercise induced
Tight casts/dressingsa
Crush injurya
Pneumatic antishock garmentsa
The use of traction and the position of a limb affect tissue pressures. Shakespeare et al. demonstrated that leg compartment pressures were proportional to the amount of calcaneal traction that was applied. There was a rise in pressure within the deep posterior compartment of >5 % for every 1 kg added [27]. The position that the ankle is immobilized affects intracompartmental pressures. Plantar flexion elevates pressure in the anterior compartment, while dorsiflexion raises pressures in the posterior compartments; the elevations in the deep and anterior compartment pressures can fluctuate upwards of three- to sevenfold. Positioning the ankle between 0 and 37° of flexion is most protective against elevated pressures in both the anterior and deep posterior compartments [28].
Studies have evaluated the changes in compartment pressures that occur during intramedullary nailing. Moed et al. found that intramedullary nailing of closed tibial fractures in a canine model increased pressures particularly in the anterior compartment [29]. The pressure increases normalized over time and were sustained in only 2 of 10 canines. Tornetta et al. found that unreamed tibial nailing caused transient intraoperative pressure elevations up to 58 mmHg, and these elevations returned to normal by the end of the procedure [30]. McQueen and Court-Brown using continuous intraoperative monitoring had similar findings [18]. Based on these studies, it seems that intramedullary nailing causes a transient increase in compartment pressures that returns to normal over time.
Polytrauma patients are at high risk for a delay in the diagnosis of a compartment syndrome compared to patients with isolated injuries [21, 31–34]. Anesthetized or intubated patients are unable to participate in a clinical exam. Other criterion (compartment measurements, pressure differentials, etc.) should be used in order to make the diagnosis in these patients.
Pathophysiology of a Compartment Syndrome
Understanding of the events that lead to the development of a compartment syndrome is essential for diagnosis and treatment. The initial inciting event leads to an increase in intracompartmental pressure. If high enough, this will decrease tissue perfusion secondary to a decreased arterial-venous gradient. Homeostasis between venous pressure, arteriolar flow, and tissue pressure is vital. Ischemic histologic changes in muscle can be seen in as little as 2 h, and changes that have clinical implications occur within approximately 3–4 h [35].
The normal relationships that exist between arterial pressure, venous pressure, and interstitial tissue pressure create an arteriovenous gradient (AV gradient) that provides adequate tissue perfusion. Since veins are collapsible, the pressure inside venules must be the same pressure as the pressure in the interstitial space. Once interstitial pressure increases, so does the pressure in the venous system. This decreases the AV gradient and tissue perfusion—this is the mechanism by which a compartment syndrome develops. Once there is an imbalance, a vicious cycle ensues where ischemia leads to further edema within the compartment that further compromises blood flow. Following the onset of ischemia, irreversible changes in nerve tissue and skeletal muscle can be seen in as little as 8 h [36] since tissue metabolic requirements are unable to be met [21–26, 31–34].
Some authors hypothesize that the difference between compartment pressure and blood pressure is vital for tissue perfusion [21, 37–41]. Hargens et al. proposed a microvascular occlusion theory where capillary occlusion is the primary factor that reduces tissue blood flow [42]. Using a canine model, they found that compartment pressures averaging 25 mmHg were sufficient to reduce tissue perfusion enough to cause capillary membrane damage resulting in an increase in permeability leading to leakage of plasma proteins which causes increased edema and decreased lymphatic drainage.
Muscle tissue can tolerate ischemia for up to 4 h before irreversible changes occur. Changes in nerves remain reversible for up to 8 h, fat up to 13 h, skin up to 24 h, and bone for 4 days. Type I and type II muscle fibers demonstrate differences in their susceptibility to ischemia. Most muscles contain a combination of red and white fibers, which are named based in the amount of myoglobin they contain. Type I, or red slow twitch fibers, rely predominantly on oxidative metabolism of triglycerides as their energy source and are particularly vulnerable to ischemia. These fibers can be found in the anterior compartment of the leg. Conversely, type II white fast twitch fibers predominantly use anaerobic metabolism of glycogen as their energy source and are more resistant to ischemia. These fibers are found in the muscles of the posterior compartment of the calf [38].
Clinical Diagnosis
Numerous papers and textbooks describe the clinical signs and symptoms of compartment syndrome. The key is a high degree of suspicion that a CS may be present and then to have the clinical acumen to diagnose it. The history, mechanism of injury, and radiographs can help identify patients at risk. The classic signs of a CS are referred to as the 6 Ps: pain out of proportion to injury, pain with passive stretch of the muscles in the involved compartments, paresthesias, pallor, paralysis, and pulselessness [26].
Pain is described as one of the earliest signs of an impending compartment syndrome, particularly pain out of proportion to the injury, or pain that was well controlled then suddenly increases. Judging pain levels can be difficult since:
Perception of pain levels varies widely from patient to patient.
Patients may have pain due to other injuries (distracting injury).
It may be impossible to determine if a patient’s pain is due to a developing compartment syndrome or to their initial/associated injury.
Patients with a neurologic injury may not be able to complain of pain.
Patients who have had regional or local blocks will not be able to complain of pain.
Patients with an altered sensorium due to intoxication or head injury may not be able to communicate adequately.
Patients who are intubated and sedated will not be able to complain of pain.
Younger pediatric patients may be unable to effectively communicate as well.
Therefore, assessing pain may be possible in an alert patient with an unaltered sensorium, but that is not always the clinical situation for a trauma patient, and other diagnostic modalities should then be considered [17, 18, 21].
Paresthesias are another early sign of CS and occur secondary to nerve ischemia. Cessation of conduction occurs after approximately 75 min of complete ischemia [43].
A “swollen or full” feeling compartment may be an early physical exam finding suggestive of compartment syndrome and may be the only detectable sign in an obtunded or unconscious patient. Although the presence of fullness should trigger the thought that a CS may be present, the degree of “fullness” is not a reliable indicator as to whether a CS is present or not. The correlation between the subjective finding of compartment “firmness” and the pressure consistent with compartment syndrome is quite poor. Shuler and Dietz reviewed physicians’ (junior/senior residents and attending surgeons) ability to manually detect elevations in intracompartmental pressures of the leg and showed overall sensitivity of 24 % with a specificity of only 55 %. The positive and negative predictive values were 19 and 63 %, respectively [44]. It seems that the presence of “firmness” should arouse suspicion for a CS, but the degree of firmness cannot and should not used to determine whether a CS is present or not.
Pallor and paralysis are late signs of a CS and indicate the presence of significant tissue damage with poor prognoses. Paralysis is a sign of irreversible nerve damage with a low probability of functional recovery. Bradley et al. reported functional recovery in 13 % of patients presenting with paralysis [45]. Pulselessness is not typically seen with a CS and usually indicates that an arterial injury is present. In order to not have a pulse, compartment pressures would need to rise close to the systolic blood pressure.
Although compartment syndromes have been recognized and treated for many years, the methods that are available for diagnosing them still have issues. It is said that in an awake, alert patient without the presence of distracting injuries, the diagnosis of a compartment syndrome is a clinical one. Although the 6 Ps have been described as the cardinal symptoms of a compartment syndrome, paralysis is a late finding, pulselessness requires very high pressure on the order of systolic blood pressure that is rarely seen, and pallor would reflect ischemia of the limb as a whole rather than individual compartments. Studies evaluating pain out of proportion to injury, “fullness” of a compartment, and paresthesias emphasize the high negative predictive value of these findings, meaning that their absence is a relatively good way to exclude a compartment syndrome. However, the positive predictive values of these signs are poor, meaning that when they are present there may or may not be a compartment syndrome. In addition, the literature is confounded by the absence of a defined, universally applied, diagnosis of what a compartment syndrome or what a missed compartment syndrome is.
Compartment Pressure Measurements
The diagnosis of compartment syndrome may be made based on the physical examination in patients who are lucid, without gross neurological compromise, who can effectively communicate, and have clear finding on a physical exam. It is more difficult to make the diagnosis in patients in whom the clinical exam is unclear or when a patient is obtunded or intubated. In these scenarios the measurement of intracompartmental pressures (CPs) may be helpful. However, CPs do not directly measure tissue ischemia, they measure tissue pressures which are used as an indirect marker of tissue ischemia.
Pressure Thresholds
There is a range of absolute pressures cited in the literature for the diagnosis of a compartment syndrome. These pressures range from 30 to 45 mmHg [46–49]. Mubarak defined 30 mmHg as the critical pressure for decompression since this is the pressure at which the perfusion of muscles and nerves is decreased. He also found that pain and paresthesias were noted by his osteotomy patients once their compartment pressures rose over 30 mmHg [50]. Matsen considered absolute pressures over 45 mmHg as an indication for a fasciotomy. He also felt that an isolated elevated pressure value was of limited utility for decision making due to individual variations in tolerance to elevated compartment pressures [49].
Unfortunately, there is no accepted absolute value at which a fasciotomy should be performed. The numbers vary due to factors including (1) individual differences in the sensitivity of tissues to ischemia, (2) the AV gradient is dependent on a patient’s blood pressure and, (3) damaged muscle is more sensitive to ischemia compared to non-damaged muscle. Because of the lack of consensus on an absolute critical pressure, relative or differential pressures are often used to aid in the diagnosis of CS.
Whitesides was the first to recommend the use of the difference between diastolic blood pressure and compartment pressure (Δ[DELTA]P). He felt that the clinical symptoms of a CS were variable and therefore not the most reliable indicator for the presence or absence of a compartment syndrome. His experimental work revealed inadequate tissue perfusion once the tissue pressure is within 10–30 mmHg of diastolic blood pressure. He recommended a fasciotomy when the ΔP reached these levels [46]. When absolute tissue pressures were in the 20–30 mmHg range, he recommended close monitoring of the patient with repeat measurements every 1–2 h. Normotensive patients with diastolic pressures of 70 mmHg and suspected compartment syndrome should be decompressed once the absolute tissue pressures reach 40–45 mmHg.
The most common pressure measurement used clinically is the difference between diastolic blood pressure and compartment pressure. Court-Brown and McQueen, based on a study where they continuously monitored compartment pressures in 116 patients with diaphyseal tibia fractures, recommended the threshold for fasciotomy when the difference between diastolic blood pressure and the compartment pressure was a Δ[DELTA]P of 30 mmHg or less (Δ[DELTA]P) [18]. Using these criteria only 3 of the 116 patients developed an acute compartment syndrome, and there were no symptoms of any missed cases seen during the follow-up period. At follow-up of 6 months, none of the patients with a Δ[DELTA]P >30 mmHg had any sequelae from a missed compartment syndrome.
Using Δ[DELTA]P requires a reliable and reproducible diastolic blood pressure. Patients under anesthesia have a decreased diastolic blood pressure (DBP) that returns to the preanesthetic level postoperatively. Therefore the preoperative and not the intraoperative DBP should be used to calculate ΔP intraoperatively to make the decision whether or not to perform fasciotomy. Tornetta et al. found an average drop in diastolic pressure of 18 mmHg +/− 13 during surgery when they reviewed 242 anesthetized patients undergoing tibial intramedullary nailing [51].
At present there is no universally accepted absolute value used to define compartment syndrome, and many clinicians use relative pressures. Controversy remains about whether the existing thresholds are reliable. Prayson et al. measured compartment pressures in patients with isolated lower extremity fractures who had no clinical signs of a compartment syndrome and used the contralateral non-injured limb as a control. The average compartment measurements in the injured leg were 35.5 mmHg versus 16.6 mmHg in the uninjured leg, and 58 % of the patients had a Δ[DELTA]P of 20. Yet, despite not having a fasciotomy, no patient had sequelae of an unrecognized compartment syndrome at 1 year of follow-up [52]. The authors suggest that the current pressure criteria used to define a compartment syndrome should be interpreted with caution and highlighted the “normal” elevations in compartment pressures that occur in the presence of a fracture.
Continuous Pressure Monitoring
While there is debate regarding critical pressures, there is a consensus that the early diagnosis of compartment syndrome is of paramount importance to prevent late sequelae. The use of continuous pressure monitoring has been investigated as a method that could be used to detect early pressure rises and therefore earlier detection and treatment of compartment syndromes. Court-Brown [18] used continuous pressure monitoring and did not miss any compartment syndromes, which suggests that continuous pressure monitoring is helpful. Harris et al. randomized 200 extra-articular tibia fractures in alert patients into a continuously monitored group (100) and an unmonitored group (100). All patients were also monitored with repeated physical examinations. Five patients in the unmonitored group developed compartment syndrome, while none in the monitored group did. At 6 month of follow-up, there were no significant differences in complication rates or late sequelae between the two groups. The authors concluded that elevated postoperative compartment pressures did not correlate with the development of a compartment syndrome, and the clinical exam by itself is sufficient to detect a CS. They also concluded that continuous pressure monitoring of tibia fractures is not indicated in awake, alert patients who can be adequately observed [53].
Methods to Measure Compartment Pressures
The most common devices used to measure pressures use either a side-ported needle or a slit catheter. A slit catheter is used for continuous pressure monitoring, while a side-ported needle is used for a static measurement. No difference has been shown between the two devices [54]. Pressure measurements using a standard 18G needle (which does not have a side port) result in higher pressures than either a slit catheter or a side-ported needle and should not be used.
There is a pressure gradient within compartments. The highest pressure is located at the fracture site and just adjacent to it. The pressure decreases the further you measure from the fracture site. Therefore, to increase the accuracy of pressure measurements, it is recommended to check pressures at multiple sites, including within 5 cm proximal and distal to the fracture [55].
Newer Noninvasive Diagnostic Modalities
Near-Infrared Spectroscopy
Due to the issues with the clinical diagnostic criteria used to diagnose CS as well as with compartment pressures, alternative diagnostic modalities are being investigated. Tissue ischemia correlates with the degree of muscle oxygenation. Shuler et al. directly measured tissue oxygenation using near-infrared spectroscopy. Near-infrared spectroscopy samples deep tissue below the skin to determine the concentration of oxygenated and deoxygenated hemoglobin. Fourteen patients with a diagnosis of compartment syndrome secondary to trauma were evaluated. Spectroscopy was used to record values in the affected extremity, and readings from the contralateral uninjured limb were used as a control. Thirty-eight compartments had pressure evidence of ischemia with a Δ[DELTA]P <10 mmHg. Near-infrared spectroscopy values in the affected anterior, lateral, deep, and superficial posterior compartments of the injured extremity were decreased by an average of 10, 10, 9, and 16 % compared with the corresponding contralateral compartments of the uninjured leg. The authors concluded that normalized near-infrared spectroscopy values decreased significantly with decreased limb perfusion pressures [56]. Near-infrared spectroscopy has potential for use in the early diagnosis of acute compartment syndrome; however, clinical trials are lacking. Technical improvements are needed since the low depth of tissue penetration is a limiting factor in its utility. More investigation is needed prior to the widespread clinical application of this device.
Metabolic Biomarkers
Metabolic biomarkers associated with muscle ischemia may be another noninvasive means to diagnose early compartment syndrome. Creatine kinase (CK), myoglobin (Mb), and fatty acid-binding protein (FABP) are markers present in skeletal muscle and may be elevated after muscle injury and necrosis. Lampert et al. found that CK values >2,000 units/L following surgery may be a warning sign for impending compartment syndrome in the anesthetized patient [57].
The Mb/FABP ratio has also been shown to be useful in identifying skeletal muscle injury. In myocardial tissue the normal ratio is approximately 5, while in skeletal muscle it is about four times higher [58]. Frequent measurements of these values following injury or fracture could theoretically detect the early stages of an impending compartment syndrome; however, these markers lack sensitivity and specificity and are not clinically useful.
Inadequate tissue perfusion leads to an anaerobic metabolism and a low pH; elevated lactate levels within an affected compartment that occur as a result may be an indicator of an early compartment syndrome. Ischemic modified albumin (IMA) has recently been identified as a marker of myocardial ischemia, is transiently decreased when skeletal muscle is ischemic, and returns to normal quickly when tissue perfusion is restored [59]. While these serologic markers may be a clue to early diagnosis, they lack reliable sensitivity and specificity, since they are usually elevated in inflammatory conditions and after trauma.
Imaging Studies
Advanced imaging studies have limited utility in the diagnosis of CS. MRIs show compartment edema; however, it is unable to differentiate ischemic muscle from generalized soft tissue inflammation secondary to trauma. Radionuclide scintigraphy has been used to evaluate myocardial perfusion. This technique was used to evaluate limb perfusion in chronic exertional compartment syndrome. Edwards et al. showed good positive and negative predictive values with scintigraphy using 99-technetium-methoxyisobutylisonitrile (99Tc-MIBI) [60]. While low cost and minimal invasiveness are strengths of this technique, its use is limited because of the lack of specificity, time needed to perform the scan, and the difficulty repeating it [61].
Standard ultrasound has been shown to be ineffective, but a relatively newer technique, pulsed phase-locked loop (PPLL), has shown promise. Initially designed to monitor intracranial pressure, PPLL transmits ultrasound waves through the soft tissues and records the reflected waves through a transducer, which detects fascial micromotion corresponding to arterial pulsations. Decreased motion is indicative of increased compartment pressure; however, there are normal variations in fascial movements, and this limitation needs further investigation. Perfusion can be evaluated by transilluminating tissue and analyzing the light backscattered by moving red blood cells (laser Doppler flowmetry—LDF). LDF has promise, but it has only been evaluated in one study of chronic exertional compartment syndrome [62].
Direct Nerve Stimulation
Sheridan et al. used direct nerve stimulation to differentiate between neuropraxia secondary to acute compartment syndrome versus a more proximal nerve injury [63]. The absence of muscular contraction with stimulation suggests pathology from elevated pressures, while if the muscle contracts there may be a more proximal injury to the nerve itself. This method is not particularly useful in monitoring of at-risk patients for compartment syndrome because of the difficulty directly stimulating a nerve. Alterations in vibratory sensation have been shown to correlate with increased intracompartmental pressures [64].
Treatment
Once the diagnosis has been made, timely decompression is necessary. Prior to going to the operating room, anything causing external compression should be removed. Intracompartmental pressures are lowered after bivalving and spreading circumferential casts and dressings which allows room for increased swelling [65, 66]. To maximize tissue perfusion, the extremity should be placed at heart level. Placing it above the level of the heart reduces arterial inflow pressure and placing it below heart level increases venous pressures [67].
Once a compartment syndrome has been diagnosed, complete decompression via a fasciotomy is mandatory. Long skin incisions are used since incompletely releasing the skin results in persistently elevated pressures. Gaspard et al. described cases where the skin continued to cause compression after limited skin incisions, but they did not measure compartment pressures [68]. Cohen et al. made long fascial incisions using short 8 cm skin incisions in posttraumatic CS, and in over 30 % of cases, the pressures remained over 30 mmHg. When the skin incisions were extended to an average of 16 cm, pressures were significantly lowered to an average of 13 mmHg [69]. The use of long skin incisions to ensure complete decompression during a fasciotomy is mandatory.
Techniques
Calf Fasciotomy
The four compartments in the calf (anterior, lateral, superficial, and deep posterior) can be released using one lateral incision or a lateral incision combined with a medial incision. Both techniques are effective. When using the dual incision technique, the anterior and lateral compartments are decompressed through a long lateral incision placed just anterior to the fibula and extending from 5 cm distal to the fibular neck to 5 cm above the tip of the lateral malleolus (Fig. 10.1). Anterior and posterior skin flaps are then raised. The intermuscular septum is identified and the lateral and anterior compartments are released by incising the fascia. The superficial branch of the peroneal nerve needs to be identified and protected where it pierces the fascia over the lateral compartment in the distal one-third of the leg. The medial incision is made 2 cm posterior to the medial tibial border (Fig. 10.2). The fascia of the superficial posterior compartment is visualized by retracting the skin posteriorly. The deep posterior compartment is released by releasing the soleal muscular leash from the medial face of the tibia. Since the musculature of the superficial posterior compartment is located proximally and the musculature of the deep posterior compartment is located distally, the medial incision needs to be long to perform an adequate decompression of both muscles.
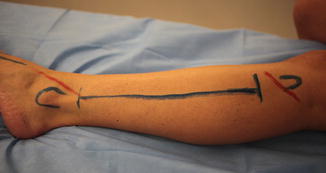
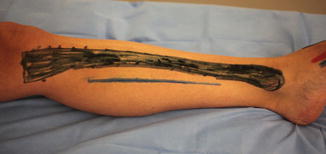

Fig. 10.1
Calf fasciotomy: the lateral incision used for a calf fasciotomy is drawn in blue. The proximal and distal ends of the fibula are outlined in blue proximal and distal to the incision. The proximal red line shows the course of the common peroneal nerve, and the distal red line shows the course of the superficial peroneal nerve in the distal calf

Fig. 10.2
Calf fasciotomy: the incision used for a medial calf fasciotomy is outlined in blue and is located 1–2 cm posterior to the subcutaneous border tibia (outlined in black). The superficial and deep posterior compartments are released using this incision when performing a two-incision technique
When a single lateral incision technique is used, the same long lateral skin incision as the two-incision technique is used. The skin is elevated off the fascia anteriorly and posteriorly, and the septum between the anterior and lateral compartments is identified, and the fascia is released. The contents of the lateral compartment are then elevated from the posterior intermuscular septum, and the superficial posterior compartment is released by incising this fascia. To decompress the deep posterior compartment, the intermuscular septum is followed down to the fibula and subperiosteal dissection of the fascia off the lateral and posterior parts of the fibula decompresses the deep posterior compartment. It is important to maintain subperiosteal dissection around the back of the fibula to avoid the peroneal vessels that are nearby and can be easily damaged.
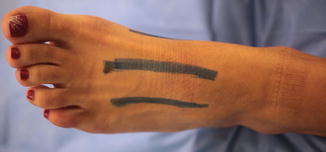
Foot Fasciotomies
Foot fasciotomies are performed using two longitudinal dorsal incisions placed just medial to the second metatarsal and lateral to the fourth metatarsal (Fig. 10.3). It is important to dissect between the metatarsals to completely release the fascia of the intrinsic muscles of the foot and achieve adequate decompression. Manoli and Weber described 9 ft compartments (medial, lateral, superficial central and deep, four interosseous, and calcaneal) and advocated making an accessory medial incision placed just plantar to the first metatarsal to release the medial and central compartments (Fig. 10.4) [70]. Others prefer using the extensile medial approach of Henry to release all compartments [71]. Care must be taken to preserve the medial neurovascular bundle with this approach.