Fig. 3.1
Roles of collagen enzymatic and nonenzymatic AGEs cross-links. The strength of collagen fibers, aggregation of collagen molecules, is determined by collagen cross-links, which bond adjacent molecules. In an analogy with building structures, collagen cross-links correspond to beams that connect reinforcing bars. Collagen cross-links can be classified into two types: enzymatic cross-links that increase bone strength and detrimental, nonphysiological AGEs cross-links that weaken the bone
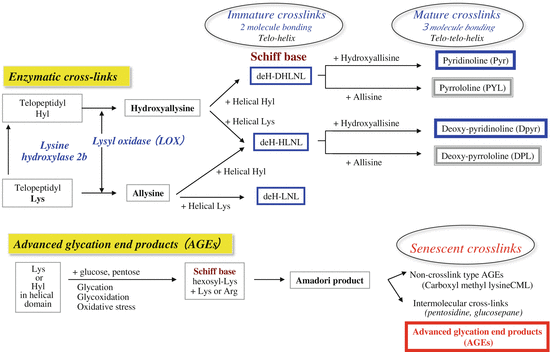
Fig. 3.2
Biochemistry of collagen cross-links

Fig. 3.3
Mechanism of reduction in bone strength. Bone quality is determined by bone material qualities and structural properties (microstructure). Sex hormone deficiency, aging, and lifestyle-related diseases induce not only reduction in bone mineral density (BMD) but also reduction in bone quality, particularly involving an AGE increase in collagen. Thus, bone strength is negatively affected. BMD and bone microstructure are parameters of bone strength that are dependent on bone remodeling. Material properties among bone quality factors are inhibited by the level of cellular function and the environment of the matrix surroundings (oxidative stress and glycation level)
3.3 Bone Quality Estimation
Oxidation and glycation increase when there is primary osteoporosis [12–14] (Fig. 3.4a), diabetes [15] (Fig. 3.4b), or renal failure [16] (Fig. 3.4c). In these diseases, we have shown that reduced formation of enzymatic cross-links and excessive AGE formation are induced in bone collagen, resulting in reduced bone strength. It has also been reported that AGEs increase in bone collagen with aging and bone strength decreases [2–4, 17]. Bone remodeling is increased due to aging and reduced sex hormones in both men and women. When bone remodeling is increased, collagen metabolism greatly increases. Therefore, it was unexpected that AGEs increase in bone collagen because AGEs are formed in proteins with long life-spans. However, there are factors related to AGE formation other than the life-span of matrix. If there is an environment that increases oxidation, glycation, or carbonyl stress (such as aging and lifestyle-related diseases), AGE formation is easily induced even with increased remodeling and shortened collagen life-span [2]. Not surprisingly, if bone remodeling is decreased and oxidation and glycation are increased, AGE formation is markedly increased in bone collagen. Diabetes corresponds to this type of pathological condition [15]. The aforementioned findings indicate that the assessment of bone fragility needs not only to measure calcium-based parameters and bone metabolism markers reflecting bone remodeling but also to simultaneously evaluate the deterioration of bone quality. From such a perspective, one needs to understand the mechanism of detrimental AGE cross-linking and beneficial enzymatic cross-linking in collagen [2–4].
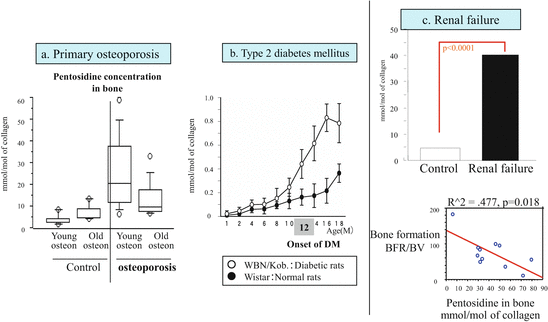

Fig. 3.4
AGE cross-linking pentosidine in bone collagen. (a) Excessive pentosidine formation in bone collagen in patients with primary osteoporosis. Bones were analyzed by dividing them into those with young osteons and those with old osteons. In a patient group with bone fractures, pentosidine was also increased even in young osteons, and AGE formation was seen from early stages of bone formation (References [12, 13]). (b) Collagen cross-links and bone strength in spontaneously diabetic rats. Pentosidine increased with progression of diabetes (−○-: WBN/Kob rats, −●-: Wistar rats). *p < 0.05: Comparison of age-matched controls and Wistar rats [15]. (c) AGE pentosidine concentrations in bone collagen and bone morphometry in hemodialysis patients. In hemodialysis patients, pentosidine levels increased markedly in bone, and the rate of bone formation decreased with such increase [16]
3.4 Roles of Enzymatic Cross-Links in Bone (Figs. 3.1 and 3.2)
The formation of enzymatic cross-links is strictly regulated by the expression of enzyme lysyl oxidase during collagen maturation and promote mineralization. The total number of such cross-links is dependent on the activity of lysyl oxidase, an enzyme secreted by osteoblasts themselves [6–8]. This cross-link formation plateaus before osteoid mineralizes [8]. If the enzyme activity is not sufficiently increased before osteoid mineralization, formation of enzymatic cross-links is decreased, and collagen fibers with sufficient strength cannot be formed [15]. We have shown that enzymatic cross-links were decreased 25 % when there was deficiency of vitamin B6 , which is an essential cofactor for lysyl oxidase activity, and that bone strength was reduced without a decrease in BMD in healthy rats [18] and diabetic rats [15]. In another study, we examined a rat model of glucocorticoid-induced osteoporosis in which bone fracture risk increases before BMD decreases. Enzymatic cross-link formation decreased due to the inhibitory effect of glucocorticoid on lysyl oxidase, and bone strength decreased despite high BMD [19]. These results indicate that enzymatic cross-links are beneficial cross-links that positively affect bone strength. In a physiological environment, the total number of enzymatic cross-links peaks in human bone from childhood to 30 years of age, and there is no excessive induction of such cross-linking [20, 21].
3.5 Roles of AGEs in Bone (Figs. 3.1 and 3.2)
AGE formation is induced by increased oxidative stress and carbonyl stress and persistent hyperglycemia. Pentosidine and glucosepane are common AGE cross-links [2]. Bone tissue analysis has shown that the amount of pentosidine formed is positively correlated with the total amount of AGEs and that pentosidine can be used as a surrogate marker of overall AGEs [2]. They form in proteins with long life-spans because AGEs form in a time-dependent manner in a physiological environment [2, 12, 13, 20]. Pentosidine increases in bone collagen with aging, and bone strength decreases [2]. If patients have diseases that increase glycation or oxidation, excessive AGE formation occurs, which greatly exceeds time-dependent AGE formation [2–4, 14–16]. AGEs decrease bone strength in two ways. One way is by directly affecting cross-link formation [2–4]. The other way is by decreasing osteoblast function via cell surface receptors for AGE (RAGE) and by decreasing biological function through induction of apoptosis [22, 23]. When excised bone block was incubated by sugar solution to induce AGE cross-linking, the strength of these bone blocks was reduced, which is independent of BMD. Thus, bone strength is decreased not only with decreased cell function but also with excessive cross-linking alone due to AGEs. This result is important in better understanding deterioration of bone quality [24].
3.6 Age-Related Changes in Pentosidine Concentration in Bone, Serum, and Urine
Urinary [10] and serum [25] pentosidine levels increase with age just as pentosidine levels in bone collagen increase with age. The age-related AGE increase in bone is a phenomenon observed in both men and women [12, 13, 17, 20, 26].
Currently, the established methods of pentosidine measurement are precision instrumental analysis using high-performance liquid chromatography (HPLC) and enzyme-linked immunosorbent assay (ELISA) using antibodies against pentosidine. Measurements using ELISA are covered by the Japanese national health insurance as a routine test for patients with renal impairment (measured at SRL, Shinjuku-ku, Tokyo, Japan). In one study, our laboratory examined the correlation between pentosidine levels in bone collagen and serum and urinary pentosidine levels. In this study, samples from orthopedic surgery patients (n = 100) were used, and correlation was examined between measurements by HPLC and measurements by ELISA [27]. The results showed that pentosidine levels measured by HPLC were positively correlated among samples of bone collagen, blood, and urine. However, plasma pentosidine levels measured by ELISA were not significantly correlated with urinary or bone pentosidine levels measured by HPLC. One reason is that current ELISA method uses heat treatment as pretreatment for pentosidine measurement [28]. It has been indicated that AGEs such as pentosidine and carboxymethyllysine are increased as artifacts when heat treatment is used. Artifacts do not occur in acidic conditions even when heat treatment is used, and improvement in the pretreatment method should be considered based on such a finding [28]. Improved ELISA methods are currently being developed that enable measurement without heat treatment, and there is a potential in this method.
3.7 Bone Quality Markers: Pentosidine and Homocysteine
It is known that decreased enzymatic cross-linking and increased pentosidine formation in bone occur in primary osteoporotic patients (15–25 patients) with femoral neck fractures [2–4] (Fig. 3.4a). This result was consistent with the finding in our collaborative study with Shiraki et al. in which a high urinary pentosidine concentration was a fracture risk factor independent of BMD [10]. In the study of Shiraki et al., 432 postmenopausal women untreated for osteoporosis were examined longitudinally with the end point of incident vertebral fracture. The results showed that the highest quartile group of urinary pentosidine (creatinine correction: 47.5 pM/mg Cr) was a fracture risk factor (odds ratio: 1.3) independent of BMD, age, bone metabolism markers, prevalent fracture, and renal function (creatinine clearance) (Fig. 3.5). This risk was higher than that for BMD, a traditional fracture risk factor . In another report, we showed that serum homocysteine levels were high in patients with high pentosidine levels in bone collagen [13]. Homocysteine inhibits lysyl oxidase activity and causes decreased formation of beneficial cross-links. Simultaneously, homocysteine increases oxidative stress, which promotes AGE formation in collagen [14]. Mild hyperhomocysteinemia has been shown to be a fracture risk factor independent of BMD in studies such as the Rotterdam study [29], Framingham study [30], and Women’s Health Initiative (WHI) cohort study [31]. Thus, hyperhomocysteinemia began to be considered a factor that reduces bone quality [2, 4, 32]. In a recent study, meta-analysis showed that hyperhomocysteinemia is a fracture risk factor independent of BMD in both men and women [33].
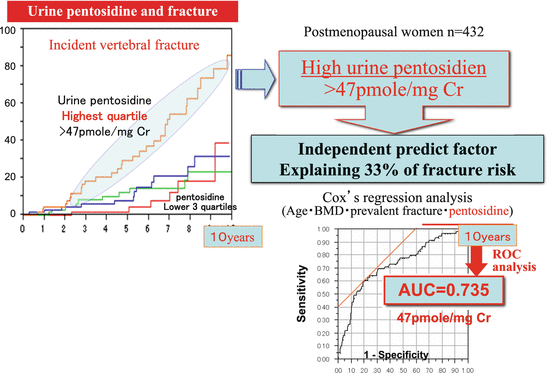

Fig. 3.5
Urinary pentosidine levels and fracture risk. A prospective study was conducted on 432 postmenopausal women using incident fracture as an end point. The results showed that the highest quartile group of urinary pentosidine (creatinine correction: 47.5 pM/mg Cr) was a fracture risk factor independent of BMD, age, bone metabolism markers, prevalent fracture, and renal function (creatinine clearance). In addition, 93 % of incident vertebral fractures could be explained by BMD, prevalent fracture, age, and urinary pentosidine concentration, and a high urinary pentosidine level was shown to be a predictor of fracture, which was attributed 33 % of the fracture risk (AUC = 0.735) [10]
In one study, we used ovariectomized rabbits to examine whether hyperhomocysteinemia induces AGE formation in bone collagen [14]. The results showed that when hyperhomocysteinemia was induced using a 1 % methionine diet, decreased enzymatic cross-linking in bone collagen and increased pentosidine formation were induced, and bone strength decreased without a decrease in BMD. Even in a general population, high serum homocysteine levels induce cross-link abnormalities in bone collagen and are thought to increase the fracture risk by reducing bone quality [2]. Recently, the OFELY study indicated that many fracture events occurred in patients with high urinary pentosidine levels, and a replication study was performed to investigate the importance of bone quality assessment [34].
3.8 Other Factors for Poor Bone Quality: Diabetes and Chronic Kidney Disease
In meta-analysis, Vestergaard showed that fractures occur in patients with type 2 diabetes and high BMD, and deterioration of bone quality began to be considered as the cause of fractures [35]. The authors of the present article examined spontaneously diabetic WBN/Kob rats . The results showed that when persistent hyperglycemia and vitamin B6 deficiency due to impaired insulin action develop, enzymatic cross-linking decreases and AGE cross-linking increases in bone collagen, resulting in decreased bone strength without a decrease in BMD [15] (Fig. 3.4b). Other studies have reported that high serum pentosidine levels [36] and high urinary pentosidine levels [37] become independent fracture risk factors in patients with type 2 diabetes. These results were consistent with the aforementioned relationship between pentosidine increase in bone collagen and decrease in bone strength. The authors of the present article found that pentosidine levels increase in bone collagen when oxidative stress increases with increased AGE formation in collagen and when renal impairment occurs, which increases oxidative and carbonyl stress [16] (Fig. 3.4c). In addition, the authors showed that osteoblast function decreased as AGE formation increased in bone collagen [16]. Clinical application of bone quality markers can also be expected in patients with diabetes and renal impairment [2–4].
3.9 Classification of Osteoporosis by BMD and Bone Quality Markers (Fig. 3.6)
The increased fracture risk associated with aging cannot be explained by decreased BMD alone or by decreased bone quality alone. The degrees to which BMD and bone quality decrease vary by individual. In one study, our laboratory examined 502 postmenopausal women (Nagano cohort study) and assessed the fracture risk by type of bone fragility. Bone fragility of each woman was classified into one of the following three types based on BMD and bone quality [6] (Fig. 3.6): osteoporosis with low BMD, osteoporosis with low bone quality, and osteoporosis with a combination of both types (osteoporosis with low BMD + low bone quality). Patients with low BMD osteoporosis have BMD of <70 % of young adult mean (YAM). Patients with low bone quality osteoporosis have high serum levels of homocysteine or urinary pentosidine. When the fracture risk was compared with patients who had BMD of >80 % of YAM, the fracture risk was increased 3.6-fold in patients with low BMD osteoporosis and was increased 1.5-fold in patients with low bone quality osteoporosis. It was increased markedly at 7.2-fold in patients with low BMD + low bone quality osteoporosis due to their synergistic effect. The ratio of patients by type was 5:3:2 for low BMD type, low bone quality type, and low BMD + low bone quality type, respectively, showing that the low bone quality type was not rare.
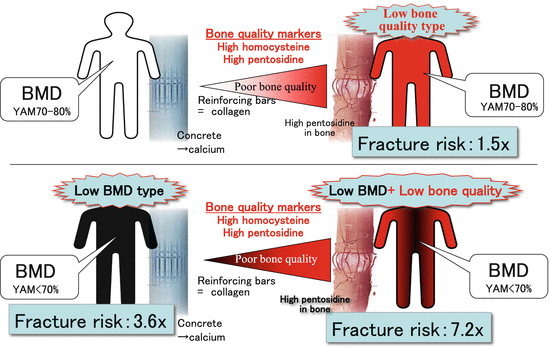

Fig. 3.6
Classification of osteoporosis by BMD and bone quality markers (homocysteine and pentosidine). Increases in fracture risk in osteoporosis were divided into three patterns using the finding that hyperhomocysteinemia causes AGE (pentosidine) accumulation in bone. “Low bone quality osteoporosis” is a condition in which fracture risk increases with the presence of abnormal homocysteine metabolism alone, even if the BMD is ≥70 % of young adult mean (YAM). “Low BMD osteoporosis” is a condition in which fracture risk increases due to low BMD even if homocysteine metabolism is satisfactory. “Low BMD + low bone quality osteoporosis” is a condition in which both BMD and homocysteine metabolism decrease. The fracture risk was increased 1.5-fold in patients with low bone quality osteoporosis, 3.6-fold in patients with low BMD osteoporosis, and 7.2-fold in patients with low BMD + low bone quality osteoporosis. YAM young adult mean BMD (Modified from Shiraki et al. [9])
3.10 Potential of Therapeutic Drug Use Based on BMD and Bone Quality Markers
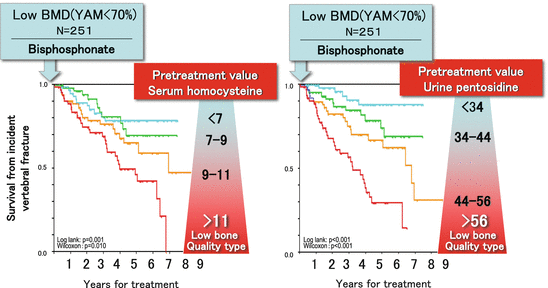
In a recent study, the authors of the present article have shown that drugs for osteoporosis can be used based on osteoporosis type classified by BMD and bone quality [38]. Bisphosphonate , anti-resorption, was administered to 251 postmenopausal patients with osteoporosis, and factors were analyzed which affected incident bone fractures after drug administration began. The study examined BMD, bone resorption and formation markers, presence or absence of prevalent fracture, age, and bone quality markers (plasma homocysteine and urinary pentosidine) at baseline. The results showed that independent risk factors for incident fracture were a high plasma homocysteine level and a high urinary pentosidine level at the commencement of treatment. Even when BMD was increased, the risk for incident fracture was increased 1.6-fold in patients with low BMD + low bone quality osteoporosis (Fig. 3.7). Bisphosphonates inhibit bone resorption and increase BMD but do not affect the formation of enzymatic cross-links in bone collagen [38]. In addition, bone collagen renewal is inhibited when metabolism is excessively inhibited long term by bisphosphonates. Then AGE cross-links increase in a time-dependent manner and microcracks develop in bone. Thus, it is important to monitor the level of bone metabolism over time using bone resorption and formation markers in clinical practice. The aforementioned findings indicate the need to improve the quality of bone collagen in addition to the importance of increased BMD in patients with low BMD + low bone quality osteoporosis [5].